Studies report nephrotoxicity during polymyxin therapy against
Abstract
Nosocomial infections caused by carbapenem-resistant Acinetobacter baumannii (CRAB) have become a global concern. The extensive antibiotic resistance of CRAB has significantly limited treatment options, while its prevalence in hospital outbreaks has amplified infection rates. This scenario has led to a resurgence of interest in polymyxins, an older class of antibiotics previously overlooked due to perceived toxicity. Polymyxins, cationic polypeptide antibiotics, now represent a last-resort treatment option. Despite their historical use, modern assessment methods have only recently been applied to evaluate polymyxins. Two polymyxins are available for clinical use: polymyxin B and colistin (polymyxin E). Notably, the administration of these drugs is hindered by toxicities, primarily nephrotoxicity and neurotoxicity, alongside less common adverse effects such as injection pain, hypersensitivity reactions, and bronchospasms.
Keywords
- Acinetobacter baumannii
- polymyxin
- toxicity
- nephrotoxicity
- neurotoxicity
1. Introduction
Antimicrobial resistance (AMR) has escalated into a global healthcare crisis, rendering many pathogens resistant to current treatments [1]. A comprehensive analysis estimated 1.27 million deaths attributable to bacterial AMR in 2019 [2], and projections indicate that 2050 annual AMR-related deaths could reach ten million [3].
Over the past three decades,
Managing
A significant subset of CRAB isolates is extensively drug-resistant (XDR; i.e., non-susceptible to ≥1 agent in all but ≤2 classes) or pan drug-resistant (PDR; i.e., non-susceptible to all antimicrobial agents have been reported worldwide) [10, 11, 12], compounding the challenge. Limited effective antibiotic options against CRAB pose a substantial health challenge. Polymyxins, though previously overshadowed, regained prominence in the late 1990s due to their activity against carbapenem-resistant (CR) infections [13]. However, new-generation antimicrobials, particularly β-lactam/β-lactamase inhibitors, have largely replaced polymyxins in CR Gram-negative bacterial infections. Conversely, polymyxins are vital for tackling resistant pathogens [13, 14, 15], especially where new agents are unavailable [16]. Nonetheless, they come with adverse effects, including allergic reactions, neurotoxicity, and nephrotoxicity [17].
2. Polymyxins
2.1 History of discovery
Polymyxins are cationic polypeptide antibiotics derived from
2.2 Structure
Polymyxins’ structure resembles antimicrobial peptides deployed by eukaryotes against pathogens. They are natural non-ribosomal cyclic lipopeptides weighing around 1.2 kDa (Figure 1) and consist of a cyclic ring of amino acids with a tripeptide chain, which binds to the lipid part of the molecule. The decapeptide core of polymyxins contains an intramolecular loop of starch-linked heptapeptides between the amino group on the side chain of the aminobutyric acid (Dab) residue at position four and the carboxyl group on the C-terminal threonine residue. They also have several other distinctive structural features, including five non-proteogenic Dab residues positively charged at physiological pH, conserved hydrophobic residues at positions 6 and 7, and an N-terminal acyl group [31]. The cationic peptide ring of these antibiotics is the same between the two polymyxins, except for a single amino acid: a D-Leu from colistin is relocated by D-Phe to polymyxin B [14, 26, 27, 29, 30, 31, 32]. However, the pharmacokinetics of polymyxin B and colistin differ notably due to the different pharmaceutical forms in which they are administered—active and prodrug form, respectively [33]. Its mechanisms of action occur through the rupture of the external and cytoplasmic membranes of the bacteria, causing loss of the contents of the cell’s interior [34]. Polymyxin B comprises at least four components and polymyxin B1 to B4, which differ only in the portion containing fatty acids, polymyxin B1 and B2 being in greater proportion [35].
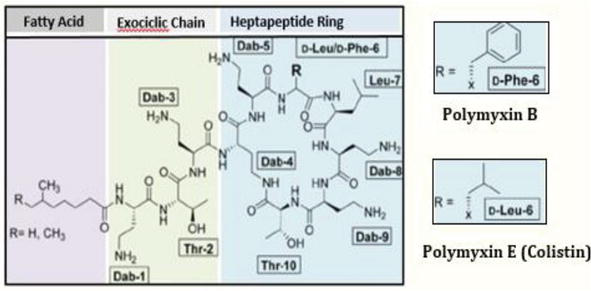
Figure 1.
Cyclic lipopeptide structure of polymyxin B (1). Colistin (polymyxin E) features a substitution of one (D-Leu) with one (D-Phe) (2).
2.3 Mechanism of action
Polymyxins exert rapid bactericidal effects by interacting with lipopolysaccharides (LPS) in the bacterial outer membrane, inducing disruptions that compromise membrane integrity. LPS, a critical component of the bacterial outer membrane, encompasses the O antigen, polysaccharide core, and lipid A. The positive charge of the polymyxin ring facilitates its binding to the outer membrane’s lipid A, leading to the displacement of stabilizing Mg2 and Ca2 ions, which is crucial for LPS integrity [35]. The fatty acid side chains also engage with LPS, enabling the secure insertion of polymyxin into the outer membrane. This interaction triggers a series of detrimental effects, including changes in outer membrane permeability, leakage of cell contents, and eventual bacterial cell death [29, 36]. Beyond inducing cytoplasmic leakage, this binding may neutralize the biological properties of endotoxins [14, 29]. Multiple hypotheses and models exist to explain the various mechanisms underlying polymyxin’s bactericidal activity [13, 14, 29]. The principal pathways through which polymyxins exhibit their activity are shown in Figure 2.
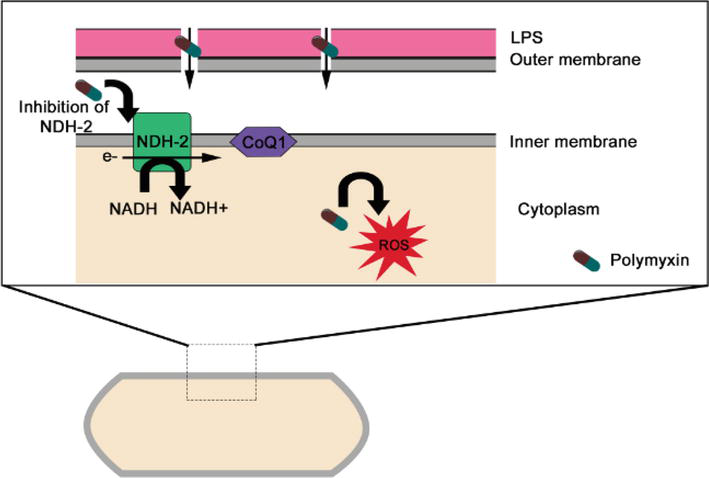
Figure 2.
Mechanisms of antibacterial activity of polymyxins in gram-negative bacteria. Disruption of the outer membrane, vesicle-vesicle contact, inhibition of respiratory enzyme NDH-2, and hydroxyl radical formation. CoQ1, coenzyme Q1.
2.4 Polymyxin resistance
The resistance of microorganisms to polymyxin remains incompletely understood, potentially arising from mutation or adaptation mechanisms [37, 38]. In most Gram-negative bacteria, the PhoP/Q and PmrA/B regulatory systems are pivotal in mediating polymyxin resistance. These systems oversee mechanisms that induce chemical modifications in the structure of bacterial lipopolysaccharides (LPS) (Figure 3). In response to low levels of antimicrobial peptides, Mg+2 and Ca+2 ions, as well as other inducers such as low pH, excessive Fe+3, excessive Al+3, and phagosomes, these systems modulate resistance by altering the cationic charge of the cell wall. Cumulatively, these modifications reduce the negative charge of the bacterial outer membrane, resulting in a diminished affinity of polymyxin for the bacterial cell surface [29].
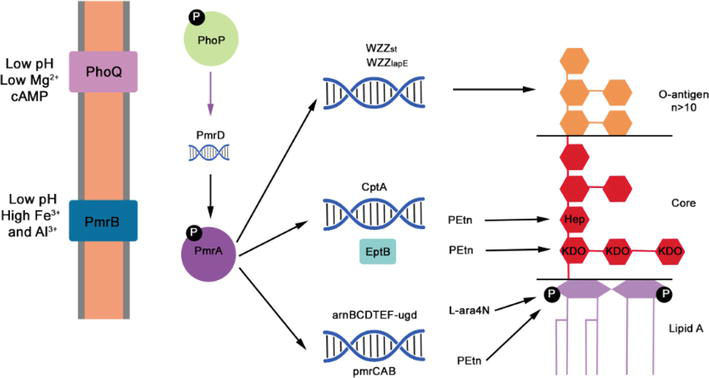
Figure 3.
Mechanism of polymyxin resistance changes in LPS. The PhoQP two-component system triggers pmrD expression. PmrD activates PmrA, cptA pmr, and the am operon. Working alongside EptB, CptA brings about modifications in the core polysaccharide of LPS. The pmr and am products facilitate the substitution of lipid a phosphates by Petn and L-ara4N, respectively. These collective alterations influence the charge of the outer membrane, resulting in polymyxin repulsion.
Modifying lipid A within the lipopolysaccharide (LPS) molecule, catalyzed by the gene products of pmrCAB and arnBCADTEF, is a fundamental mechanism underlying bacterial resistance to polymyxin antibiotics. These gene products play a pivotal role in altering the surface charge and permeability of the bacterial outer membrane (OM) [39, 40, 41].
In
Another
Mutations within the gene responsible for glycosyltransferase, a component involved in LPS biosynthesis, have also been linked to polymyxin resistance [50, 51]. According to current literature, both resistance mechanisms negate polymyxin-triggered bacterial death by obstructing the interaction of polymyxins with OM. The mechanisms are governed by the pmrCAB operon (for lipid A modification with PEtN), naxD (for galactosamine modification), or the lpx biosynthetic cluster (for LPS loss) [42, 44, 45, 46].
The outer membrane lipoprotein VacJ is an integral part of the Vps-VacJ ABC transporter system, responsible for maintaining the presence of phospholipids and LPS within the outer membrane [52]. Mutations within the vacJ and pldA genes could contribute to
2.5 Heteroresistence
Heteroresistance refers to the emergence of resistance to a specific antibiotic within a population initially sensitive to that antibiotic based on
Detecting heteroresistant strains necessitates using the population profile analysis (PAP) method, the gold standard for identifying heteroresistance. In clinical practice, the introduction of the mini-PAP method, particularly for colistin with MIC >2 mg/L, has been recommended [73]. However, the fact that conventional susceptibility testing categorizes heteroresistant isolates as susceptible to colistin poses a notable concern [65]. Heteroresistance can sometimes be indicated by colonies within the growth inhibition zone, as seen with Etest® strips or disc diffusion assays. Nevertheless, standard dilution methods used for MIC determination fail to detect heteroresistance, potentially leading to suboptimal patient dosages. This suboptimal treatment might inadvertently select the resistant population, contributing to therapeutic failures [26, 74]. Inappropriate colistin use also holds significant potential for rapid resistance development and therapeutic inefficacy [75]. Under selection pressure, a subpopulation of resistant cells within a heteroresistant population can become predominant, yielding an entirely resistant population [68].
2.6 Clinical use
In clinical practice, polymyxins are employed as either polymyxin B or colistin. Despite their structural similarity, these drugs differ in their administered forms and exhibit distinct clinical pharmacokinetics (PK) [30]. Polymyxin B is directly administered in its active form as polymyxin B sulfate salt. In contrast, colistin is administered as an inactive prodrug called colistin metasulfate or colistimethate (CMS). Once metabolized, CMS is converted into the active ingredient colistin base. CMS is less toxic than colistin, and its conversion to colistin occurs gradually, coupled with rapid renal elimination.
Consequently, only about 20–25% of the administered CMS is effectively transformed into colistin [76, 77, 78]. Polymyxin B administration leads to quicker attainment of target concentrations [79]. Although polymyxin B and colistin exhibit comparable in vitro antimicrobial activity [30], differences in their plasma concentration profiles following therapy initiation will likely significantly impact their pharmacodynamic responses in patients.
3. Polymyxin toxicity
The 1990s saw the emergence of multidrug-resistant bacteria, including those resistant to β-lactams, aminoglycosides, and quinolones, causing nosocomial infections, particularly in intensive care units [80, 81, 82, 83]. This scenario increased interest in polymyxins and spurred several reviews [84, 85]. These drugs’ most significant adverse effects include nephrotoxicity, particularly acute renal failure, and neurotoxicity. The latter is thought to result from the high binding affinity of polymyxins to brain and renal tissues [86]. Additional effects encompass allergies leading to skin lesions resembling urticaria, pain at the injection site (with intramuscular administration), thrombophlebitis (with intravenous injection), fever, and eosinophilia [87, 88].
3.1 Nephrotoxicity
Nephrotoxicity ranks as the foremost adverse event often linked to the use of polymyxins. Thus, comprehending the mechanisms and risk factors for its development has been a focal point of research [89, 90]. Clinical manifestations of polymyxin-associated nephrotoxicity include direct toxicity to renal tubules leading to tubular necrosis, oxidative damage, decreased glomerular filtration rate, reduced creatinine clearance, and elevated serum urea and creatinine levels [80, 91]. Risk factors for kidney damage among polymyxin users encompass high doses, concurrent use of other nephrotoxic drugs, vasoactive medication requirements, and a higher body mass index [92, 93, 94, 95]. The substantial concern with nephrotoxicity lies in its dose-dependent nature. In other words, the choice of therapy can influence the extent of drug-induced toxicity, potentially exacerbating the clinical condition of patients [96]. Dose-dependent nephrotoxicity is the most frequently reported adverse event with intravenous polymyxin use, affecting between 30 and 60% of patients [78, 85, 97, 98, 99, 100, 101]. However, it is often reversible [102]. While most studies have examined colistin, fewer studies have focused on polymyxin B. Due to the slower conversion of CMS to colistin, reaching therapeutic serum levels may be delayed, necessitating higher initial CMS doses to achieve effective treatment early on. However, this strategy is constrained by the potential for nephrotoxicity. Polymyxin B, administered directly in its active form, reaches the desired plasma concentration more promptly [30]. Recent literature suggests greater nephrotoxicity with colistin compared to polymyxin [103]. However, these findings require careful evaluation due to many factors influencing nephrotoxicity development, especially during the initial stages. Additionally, the potential nephrotoxicity of low polymyxin B doses may have been underestimated. Several studies have explored the efficacy of polymyxin B and colistin against
| N° of patients/therapy | GNB (n) | Definition of nephrotoxicity | Nephrotoxicity (%) | Mortality rate (%) | Ref. |
|---|---|---|---|---|---|
| 60/COL | AB (39) PA (21) | CrL of 1.5 mg/dL or urea level of 50 mg/dL | 27 (NRF) 58 (ABCL) | 37 | [104] |
| 21/IVCOL | AB (21) | SCr value of 12 mg/dL, reduction in the calculated CLCr of 50% relative to the matter at antibiotic therapy initiation, or a decline in RF that resulted in the need for RRT | 24 | 61.9 | [105] |
| 60/PB | AB (46) PA (2) AB + PA (2) NI (10) | Double the SCr for a value ≥2 mg/dL | 14 | 20 57 (DRF) 15 (NDRF) | [106] |
| 26/COL | PA (20) AB (6) | ND | 14.4 | 33.3 | [107] |
| 16/IVCOL, AEROPB + AA | AB (16) PA (12) | Doubling of SCr | 6 | 21 (EOT) 48 (AD) | [108] |
| 19/IVCOL | PA (12) AB (5) | CrV at the beginning of COLtreatment was compared with the maximum value of creatinine during therapy as well as with the CrV at the end of treatment using a non-parametric test (Wilcoxon) | 0 | 41.2 | [109] |
| 55/COL | AB (36) PA (19) | SCr value of 12 mg/dL, reduction in the calculated CLCr of 50% relative to the matter at antibiotic therapy initiation, or a decline in RF that resulted in the need for RRT | 0 | 27 | [110] |
| 43/COL | PA (35) AB (8) | Acute RF was defined as a rise of 2 mg / dL in the SrCr level of patients with previously normal renal function | 62.5 | 27.9 | [111] |
| 51/COL | AB (28) PA (23) | Normal renal function was defined as a SCr level of 1.3 mg/dl or lower. | 8 | 24 | [112] |
| 37/IVPB, PBVN, both (IPB/PBVN), DOXI | AB (37) | Increase in SCr of 0.5 mg/dL, or increase ≥50% in SCr or reduction of ClCr ≥50% | 21/6 | 27 | [113] |
| 45/IVPB | PA (20) AB (19) PA + AB (2) NI (4) | Acute increase in SCr level by >0.5 mg/dL over 24 h | 4 | 52 (IH) | [114] |
| 16/PB | PA (8) AB (5) KP (3) EC (1) | Increase in SCr of 0.5 mg/dL or a 50% reduction in CLCr | 55 | 63 | [98] |
| 82/COL, PB | AB (82) | Doubling of SCr (any time during treatment compared with the start of therapy) or increase by 1 mg/dL if initial SCr was 1.4 mg/dL | 26 (COl group) 27 (PB group) | 56 (COL group) 61 (PB group) | [115] |
| 114/IVPB | PA (95) AB (13) KP (1) PA + AB (2) NI (3) | Baseline SCr < 1.5 mg/dL when SCr levels increased to 1.8 mg/dL (AKI) or baseline SCr 1.5 mg/dL when SCr levels increased to >50%, or there was a need for dialysis | 22 AKI/NS | 61.4 92 (DAKI) 53 (NDAKI) | [116] |
| 276/PB | PA (126) AB (86) NI (64) | MRI: 50% but <100% (increase in creatinine concentration during therapy); MORI: 100% (increase in creatinine concentration but with no need for hemodialysis); SRI: need for hemodialysis during therapy | 15.7 (MRI) 38.3 (MOSRI) | 60.5 (IH) | [99] |
| 80/PB (NPD or CD) | KP (49) AB (21) PA (14) EC (4) ECO (1) | Defined by RIFLE criteria | 40 (1 week after the last dose) | 15 vs. 20 (EOT) 30 vs. 38 (EOH) | [116] |
| 173/COL, PB | AB (107) PA (46) | Defined by RIFLE criteria | 60 (COL group) 41.8 (PB group) | ND | [92] |
| 32/IVPB | AB (26) PA (1) ECO (1) SE (1) Mu (3) | Defined by RIFLE criteria | 18.7 | 28.1 (EOT) | [117] |
| 225/IVCOL, PB | PA (103) AB (74) KP (52) ECO (11) Other (17) | Prevalence of nephrotoxicity within 30 days in colistimethate group compared with PB group Comparison of nephrotoxicity prevalence in matched patients | 21.4 (COL group) 21.4 (PB group) | 55.3 (COL group) 21.1 (PB group) | [93] |
| 104/PB | AB (34) KP (25) PA (11) Mu (34) | Defined by RIFLE criteria | 14.4 | 47 | [118] |
| 132/COL, PB | AB (43) PA (22) KP (12) DI (18) NI (37) | Classified according to AKIN criteria | 20.8 (AKI/PB group) 38.9 (AKI/COL group) | 47 | [119] |
| 36/PB | A spp. (12) KP (8) PA (6) ECO (6) E spp. (5) Other (9) | Increase of 100% of SCr level from baseline | 21.4 | 44.5 | [120] |
| 410/PB | AB (150) PA (45) KP (42) ECO (5) EA (5) NI or NR (162) | Defined by RIFLE criteria | 12.7 | 42 | [121] |
| 151/ PB | KP (92) AB (32) PA (17) Other (10) | AKI: increase in SCr 1.5 times the value at PB initiation or the initiation of RRT by day 7 of PB treatment, defined by RIFLE criteria | 35.8 AKI | NS | [122] |
| 192/ IVPB | KP (92) AB (53) | Defined by RIFLE criteria | 45.8 | NS | [123] |
| 491/IVCOL, PB | AB (180) KP (55) PA (51) EA (9) ECO (5) NI (190) | Incidence of AKI by RIFLE criteria | 38.3 (COL group) 12.7 (PB group) | NS | [124] |
| 291/PB, NVPT, in vitro VCT | AB (228) PA (61) KP (14) Other (7) | Defined by RIFLE criteria | 98 of 291 | 23 | [125] |
| 112/IVCOL, PB | KP (31) AB (22) PA (19) ECO (5) NI (35) | A two-fold increase in SCr or a 50% decrease in estimated CLCr | 26.8 | NS | [103] |
| 84/IVPB, PBM, PB/CARB, CEFO/SUL | AB (81) | MRI: decrease in baseline CLCr of 50% or doubling of baseline SCr in patients with normal renal function, or an increase of baseline SCr of 50% or decrease of CLCr of 20% in patients with abnormal baseline anal function | 7.1 (RI) | 48.8 (IH) | [126] |
| 222/PB | AB (67) E (50) PA (15) Other (4) NI (86) | Defined by RIFLE criteria | 46.3 | 60.3 | [127] |
| 273/PB | KP (108) PA (74) AB (77) ECO (22) Other (9) | Defined by RIFLE criteria | 32 | 47 (ODD) 17 (TDD) | [128] |
| 183/IVCOL or ICOL, IVCOL/ICOL | Increase in SCr of ≥0.3 mg/dL in 2 days or ≥ 50% in 7 days after COL treatment without other defined causes | 13.3 | 19.1 | [129] | |
| 250/COL + MERO | AB (197) AB+KP (1) NS (52) | Classified according to AKI criteria | 30.8 | 41.6 | [130] |
| 39/IVCOL | PA (34) AB (5) EC (1) | Based on the ROC curve, the cutoff value of the colistin trough concentration that would predict nephrotoxicity was 2.02 mg/mL | 47.6 | 33.3 | [131] |
| 87/COL | AB (73) NS (14) | Increase in the SCr level by at least 50% from the baseline after≥48 h | 27.6 | NS | [132] |
| 50/COL | Defined by RIFLE criteria | 54 (MIC ≤0.5 μg/mL | NS | [133] | |
| 25/IVCOL | AB (25) | Increase in SCr to ≥1.5- fold from baseline, decrease in the estimated CLCr to <75% from baseline, or requirement for RRT | 20 | 40 (IH) | [134] |
| 163/COL | A spp. (118) PA (32) KP (7) E spp. (6) | Followed by KDIGO classification: creatinine elevation of ≥0.3 mg/dL in 48 h or ≥ 1.5 times baseline creatinine in an interval of up to 7 days | 46 | 17.8 | [135] |
| 101/COL | AB (101) | Defined by RIFLE criteria | 52.6 (LD group) 20.5 (WLD group) | 51.3 | [136] |
Table 1.
COL, colistin; PB, polymyxin; PBM, polymyxin B monotherapy; IVCOL, intravenously colistin; ICOL, inhaled colistin; AEROPB, aerosolized polymyxin B; IVPB, intravenously polymyxin B; TDD, twice daily dosing; NRF, normal renal function; ABCL, abnormal baseline creatinine levels; PBVN, polymyxin B
Acute kidney injury (AKI) is a prevalent clinical complication observed primarily in critical and hospitalized patients, characterized by the release of measurable proteins in both plasma and urine. This condition is rooted in the sudden decline of renal function, classified into risk, damage, failure, loss, and AKI stages [137, 138]. Critically ill patients suffering from AKI often face elevated mortality rates. This acute injury can progress to chronic kidney disease, defined by kidney damage and a glomerular filtration rate below 60 mL/min/1.73m2 over 3 months. Therefore, discontinuing polymyxin therapy is imperative whenever signs of renal failure are detected. Supportive care, including monitoring fluid intake, output, and electrolytes, becomes necessary when renal dysfunction is associated with polymyxin use [85].
3.2 Neurotoxicity
Neurotoxicity constitutes another undesirable consequence of polymyxin administration. Neurotoxicity related to polymyxins affects 7–27% of patients, with most cases involving concurrent renal failure [139, 140]. Symptoms of neurotoxicity encompass weakness, peripheral and facial paresthesia, ataxia, ophthalmoplegia, nystagmus, difficulty swallowing, and eyelid ptosis [88, 139, 140, 141, 142, 143, 144]. Severe manifestations include muscle blockade leading to respiratory failure, often requiring ventilatory support for 10 to 48 hours [140, 141]. Typically, these symptoms decrease upon tapering or discontinuation of the drug. The administration of colistin triggers the activation of pro-inflammatory mediators within neuronal cells [145]. Research indicates that neurotoxicity entails a complex interplay of apoptotic and inflammatory pathways. Studies involving colistin treatment (15 mg/kg/day for 7 days) revealed significant mitochondrial dysfunction in central and peripheral nervous tissues [146, 147]. Similarly, exposure to colistin (200 μM/24 h) induced apoptosis in around 50% of neuronal N2a cells in mice [145]. Further exploration using Western blotting and immunohistochemistry demonstrated that colistin-induced apoptosis in N2a neuronal cells hinges on generating reactive oxygen species (ROS) and the mitochondrial pathway [145, 148, 149]. Interestingly, co-administration of neuroprotective agents, such as curcumin and minocycline demonstrated,
3.3 Skin hyperpigmentation
Although nephrotoxicity ranks as polymyxin B’s most significant adverse reaction, another substantial side effect is skin hyperpigmentation. Polymyxin B induces this condition, which impacts psychological well-being and results in significant esthetic harm [150, 151, 152, 153, 154, 155, 156, 157, 158]. Cutaneous hyperpigmentation has been observed as a reaction to polymyxin B, affecting adults and pediatric and neonatal patients [151, 153, 154, 155]. According to cohort studies, the incidence of cutaneous hyperpigmentation attributed to this drug ranges from 8–15% [151, 152]. Cutaneous hyperpigmentation involves biochemical and immunological mechanisms, primarily associated with histaminergic receptors that stimulate melanogenesis, ultimately leading to melanin deposition in the dermis [150]. Typically, skin darkening manifests between the third and seventh days following the commencement of intravenous polymyxin B treatment. This phenomenon does not show significant disparities concerning light exposure or infection sites across patients [152]. Hyperpigmentation is often concentrated on the face and neck regions with higher melanocyte density, while the rest of the body remains unaffected during treatment [152, 154, 155, 159].
In some cases, discontinuing polymyxin B treatment reveals hyperpigmentation that can persist for months [150]. During the COVID-19 pandemic, polymyxin B treatment was administered to physicians with COVID-19 and secondary multidrug-resistant bacterial infections, resulting in hyperpigmentation on the head and neck [160]. This pigmentary disorder may be associated with AKI in critically ill COVID-19 patients [160]. Excessive accumulation of polymyxin B might contribute to aberrant hyperpigmentation in neonates and infants with immature renal function [153, 158].
4. Conclusions
In summary, this chapter presents a comprehensive review of the toxicity of polymyxins, which serve as the last resort for treating infections caused by carbapenem-resistant
Additionally, efflux pumps and the mcr-1 gene contribute to colistin resistance. The phenomenon of heteroresistance to colistin in
Acknowledgments
This research was funded by the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro/FAPERJ (#110.198-13) and the Brazilian Council for Scientific Research (CNPq, #467.488/2014-2 and 301744/2019-0). Funding was also provided by FAPERJ (#210.003/2018) through the National Institutes of Science and Technology Program (INCT) to Carlos M. Morel (INCT-IDPN).
Author contributions
Conceived and designed the experiments: K.R., T.P.G.C.; writing—original draft: K.R.; review and editing: K.R., S.G.D.-S.; funding: S.G.D.-S. All authors have read and agreed to the published version of the manuscript.
References
- 1.
Tang KWK, Millar BC, Moore JE. Antimicrobial Resistance (AMR). British Journal of Biomedical Science. 2023; 80 :11387. DOI: 10.3389/bjbs.2023.11387 - 2.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022; 399 (10325):629-655. DOI: 10.1016/S0140-6736(21)02724-0 - 3.
Thompson T. The staggering death toll of drug-resistant bacteria. Nature. 2022. DOI: 10.1038/d41586-022-00228-x - 4.
Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challen ges. Clinical Microbiology Reviews. 2017;30 (1):409-447. DOI: 10.1128/CMR.00058-16 - 5.
Ibrahim S, Al-Saryi N, Al-Kadmy IMS, Aziz SN. Multidrug-resistant Acinetobacter baumannii is an emerging concern in hospitals. Molecular Biology Reports. 2021;48 (10):6987-6998. DOI: 10.1007/s11033-021-06690-6 - 6.
Hamidian M, Nigro SJ. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii . Microbial Genomics. 2019;5 (10):e000306. DOI: 10.1099/mgen.0.000306 - 7.
Ramirez MS, Bonomo RA, Tolmasky ME. Carbapenemases: Transfor ming Acinetobacter baumannii into a yet more dangerous menace. Biomolecules. 2020;10 (5):720. DOI: 10.3390/biom10050720 - 8.
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. WHO pathogens priority list working group. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. The Lancet Infectious Diseases. 2018; 18 (3):318-327. DOI: 10.1016/S1473-3099(17)30753-3 - 9.
Ellis RC, Roberts EK, Grier JT, Fiester SE. Acinetobacter baumannii infections that are resistant to treatment: Warning signs from the COVID-19 pandemic. Future Microbiology. 2022;17 :1345-1347. DOI: 10.2217/fmb-2022-0153 - 10.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection. 2012; 18 (3):268-281. DOI: 10.1111/j.1469-0691.2011.03570.x - 11.
Piperaki ET, Tzouvelekis LS, Miriagou V, Daikos GL. Carbapenem-resistant Acinetobacter baumannii : In pursuit of an effective treatment. Clinical Microbiology and Infection. 2019;25 (8):951-957. DOI: 10.1016/j.cmi.2019.03.014 - 12.
Weinberg SE, Villedieu A, Bagdasarian N, Karah N, Teare L, Elamin WF. Control and management of multidrug-resistant Acinetobacter baumannii : A review of the evidence and proposal of novel approaches. Infection Prevention in Practice. 2020;2 (3):100077. DOI: 10.1016/j.infpip.2020.100077 - 13.
Nang SC, Azad MAK, Velkov T, Zhou QT, Li J. Rescuing the last-line polymyxins: Achievements and challenges. Pharmacological Reviews. 2021; 73 (2):679-728. DOI: 10.1124/pharmrev.120.000020 - 14.
Poirel L, Jayol A, Nordmann P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clinical Microbiology Reviews. 2017; 30 (2):557-596. DOI: 10.1128/CMR.00064-16 - 15.
Mmatli M, Mbelle NM, Maningi NE, Osei Sekyere J. Emerging transcriptional and genomic mechanisms mediating carbapenem and polymyxin resistance in Entero bacteriaceae : A systematic review of current reports. mSystems. 2020;5 (6):e00783-e00720. DOI: 10.1128/mSystems.00783-20 - 16.
Aslan AT, Akova M, Paterson DL. Next-generation polymyxin class of antibiotics: A ray of hope illuminating a dark road. Antibiotics (Basel). 2022; 11 (12):1711. DOI: 10.3390/antibiotics11121711 - 17.
Bayraktar I, Halacli B, Demirkan K, Topeli A. Polymyxin B-related neurotoxicity: A brief case report. European Journal of Hospital Pharmacy. 2023; 7 . DOI: 10.1136/ ejhpharm-2023-003786 - 18.
Ainsworth GC, Brown AM, Brownlee G. Aerosporin, an antibiotic produced by Bacillus aerosporus Greer. Nature. 1947;159 (4060):263. DOI: 10.1038/16 0263a0 - 19.
Benedict RG, Langlykke AF. Antibiotic activity of Bacillus polymyxa . Journal of Bacteriology. 1947;54 (1):24 - 20.
Stansly PG, Shepherd RG, White HJ. Polymyxin: A new chemotherapeutic agent. Bulletin of the Johns Hopkins Hospital. 1947; 81 (1):43-54 - 21.
Brownlee G, Bushby SR. Chemotherapy and pharmacology of aerosporin; a selective gram-negative antibiotic. Lancet. 1948; 1 (6491):127-132. DOI: 10.1016/s0140-6736(48)90090-7 - 22.
Shepherd RG, Stansly PG, Winterbottom R, English JP, Fellows CE, Ananenko NH, et al. Chemical studies on polymyxin; isolation and preliminary purification. Journal of the American Chemical Society. 1948; 70 (11):3771-3774. DOI: 10.1021/ja01191a068 - 23.
Brownlee G. Antibiotics derived from bacillus polymyxin. Annals of the New York Academy of Sciences. 1949; 51 (Art. 5):875-878. DOI: 10.1111/j.1749-6632.1949.tb27313.x - 24.
Jones TS. Chemical evidence for the multiplicity of the antibiotics produced by Bacillus polymyxa . Annals of the New York Academy of Sciences. 1949;51 (Art. 5):909-916. DOI: 10.1111/j.1749-6632.1949.tb27317.x - 25.
White HJ, Alverson CM, Baker MJ, Jackson ER. Comparative biological studies of polymyxin and aerosporin. Annals of the New York Academy of Sciences. 1949; 51 (Art. 5):879-890. DOI: 10.1111/j.1749-6632.1949.tb27314.x - 26.
Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clinical Microbiology Reviews. 2008; 21 (3):449-465. DOI: 10.1128/CMR.00006-08 - 27.
Yu Z, Qin W, Lin J, Fang S, Qiu J. Antibacterial mechanisms of polymyxin and bacterial resistance. BioMed Research International. 2015; 2015 :679109. DOI: 10.1155/2015/679 109 - 28.
Stansly PG, Brownlee G. Nomenclature of polymyxin antibiotics. Nature. 1949; 163 (4146):611. DOI: 10.1038/163611a0 - 29.
Trimble MJ, Mlynárčik P, Kolář M, Hancock RE. Polymyxin: Alternative mechanisms of action and resistance. Cold Spring Harbor Perspectives in Medicine. 2016; 6 (10):a025288. DOI: 10.1101/cshperspect.a025288 - 30.
Roberts KD, Azad MA, Wang J, Horne AS, Thompson PE, Nation RL. Et. Antimicrobial activity and toxicity of the major lipopeptide components of polymyxin B and colistin: Last-line antibiotics against multidrug-resistant gram-negative bacteria. ACS Infectious Diseases. 2015; 1 (11):568-575. DOI: 10.1021/acsI nfecdis.5b00085 - 31.
Falagas ME, Rafailidis PI, Matthaiou DK. Resistance to polymyxins: Mechanisms, frequency and treatment options. Drug Resistance Updates. 2010; 13 (4-5):132-138. DOI: 10.1016/j.drup.2010.05.002 - 32.
Zavascki AP, Bulitta JB, Landersdorfer CB. Combination therapy for carbapenem-resistant gram-negative bacteria. Expert Review of Anti-Infective Therapy. 2013; 11 (12):1333-1353. DOI: 10.1586/14787210.2013.845523 - 33.
Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of gram-negative pathogens: Results from the SENTRY antimicrobial surveillance program (2006-09). The Journal of Antimicrobial Chemotherapy. 2011; 66 (9):2070-2074. DOI: 10.1093/jac/dkr239 - 34.
Groisman EA, Kayser J, Soncini FC. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. Journal of Bacteriology. 1997; 179 (22):7040-7045. DOI: 10.1128/jb.179.22.7040-7045.1997 - 35.
Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. The Journal of Antimicrobial Chemotherapy. 2007; 60 (6):1206-1215. DOI: 10.1093/jac/dkm357 - 36.
Mohapatra SS, Dwibedy SK, Padhy I. Polymyxins, the last-resort antibiotics: Mode of action, resistance emergence, and potential solutions. Journal of Biosciences. 2021; 46 (3):85. DOI: 10.1007/s12038-021-00209-8 - 37.
Skiada A, Markogiannakis A, Plachouras D, Daikos GL. Adaptive resistance to cationic compounds in Pseudomonas aeruginosa . International Journal of Antimicrobial Agents. 2011;37 (3):187-193. DOI: 10.1016/j.ijantimicag.2010.11.019 - 38.
Trent MS, Ribeiro AA, Lin S, Cotter RJ, Raetz CR. An inner membrane enzyme in salmonella and Escherichia coli that transfers 4-amino-4-deoxy-L-arabinose to lipid A: Induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. The Journal of Biological Chemistry. 2001;276 (46):43122-43131. DOI: 10.1074/jbc.M1069612 00 - 39.
Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Frontiers in Microbiology. 2014; 5 :643. DOI: 10.3389/fmicb.2014.00643 - 40.
Jeannot K, Bolard A, Plésiat P. Resistance to polymyxins in gram-negative organisms. International Journal of Antimicrobial Agents. 2017; 49 (5):526-535. DOI: 10.1016/j.ijantimicag.2016.11.029 - 41.
Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. The pmrCAB operon mediates polymyxin resistance phosphoethanolamine modification of lipid A. Antimicrobial Agents and Chemotherapy. 2011; 55 (8):3743-3751. DOI: 10.1128/AAC.00256-11 inAcinetobacter baumannii ATCC 17978 and clinical isolates through - 42.
Moffatt JH, Harper M, Adler B, Nation RL, Li J, Boyce JD. Insertion sequence ISAba11 is involved in colistin resistance and loss of lipopolysaccharide in Acinetobacter baumannii . Antimicrobial Agents and Chemotherapy. 2011;55 (6):3022-3024. DOI: 10.1128/AAC.01732-10 - 43.
Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrobial Agents and Chemotherapy. 2010;54 (12):4971-4977. DOI: 10.1128/AAC.00834-10 - 44.
Chin CY, Gregg KA, Napier BA, Ernst RK, Weiss DS. A PmrB-regulated deacetylase required for lipid a modification and polymyxin resistance in Acinetobacter baumannii . Antimicrobial Agents and Chemotherapy. 2015;59 (12):7911-7914. DOI: 10.1128/AAC.00515-15 - 45.
Park YK, Choi JY, Shin D, Ko KS. Correlation between overexpression and amino acid substitution of the PmrAB locus and colistin resistance in Acinetobacter baumannii . International Journal of Antimicrobial Agents. 2011;37 (6):525-530. DOI: 10.1016/j.ijanti micag.2011.02.008 - 46.
Da Silva GJ, Domingues S. Interplay between colistin resistance, virulence and fitness in Acinetobacter baumannii . Antibiotics (Basel). 2017;6 (4):28. DOI: 10.3390/antibiotics6040028 - 47.
Lin MF, Lin YY, Lan CY. Contribution of EmrAB efflux pumps to colistin resistance in Acinetobacter baumannii . Journal of Microbiology. 2017;55 (2):130-136. DOI: 10.1007/s 12275-017-6408-5 - 48.
Girardello R, Visconde M, Cayô R, Figueiredo RC, Mori MA, Lincopan N, et al. Diversity of polymyxin resistance mechanisms among Acinetobacter baumannii clinical isolates. Diagnostic Microbiology and Infectious Disease. 2017;87 (1):37-44. DOI: 10.1016/j. diagmicrobio.2016.10.011 - 49.
Hood MI, Becker KW, Roux CM, Dunman PM, Skaar EP. Genetic determinants of intrinsic colistin tolerance in Acinetobacter baumannii . Infection and Immunity. 2013;81 (2):542-551. DOI: 10.1128/IAI.00704-12. Erratum in: Infect Immun. 2014 Jan; 82 (1):469 - 50.
Lean SS, Yeo CC, Suhaili Z, Thong KL. Comparative genomics of two ST 195 carbapenem-resistant Acinetobacter baumannii with different susceptibility to polymyxin revealed underlying resistance mechanism. Frontiers in Microbiology. 2016;6 :1445. DOI: 10.3389/fmicb.2015.01445 - 51.
Malinverni JC, Silhavy TJ. An ABC transport system that maintains lipid asymmetry in the gram-negative outer membrane. Proceedings of the National Academy of Sciences of the United States of America. 2009; 106 (19):8009-8014. DOI: 10.1073/pnas.0903229106 - 52.
Thi Khanh Nhu N, Riordan DW, Do Hoang Nhu T, Thanh DP, Thwaites G, Huong Lan NP, et al. The induction and identification of novel colistin resistance mutations in Acinetobacter baumannii and their implications. Scientific Reports. 2016;22 (6):28291. DOI: 10.1038/srep28291 - 53.
Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. The Lancet Infectious Diseases. 2016; 16 (2):161-168. DOI: 10.1016/S1473-3099(15)00 424-7 - 54.
Paterson DL, Harris PN. Colistin resistance: A major breach in our last line of defense. The Lancet Infectious Diseases. 2016; 16 (2):132-133. DOI: 10.1016/S1473-3099(15) 00463-6 - 55.
Ma F, Shen C, Zheng X, Liu Y, Chen H, Zhong L, et al. Identification of a novel plasmid carrying mcr-4.3 in anAcinetobacter baumannii strain in China. Antimicrobial Agents and Chemotherapy. 2019;63 (6):e00133-e00119. DOI: 10.1128/AAC.00133-19 - 56.
Bitar I, Medvecky M, Gelbicova T, Jakubu V, Hrabak J, Zemlickova H, et al. Complete nucleotide sequences of mcr-4.3 -carrying plasmids inAcinetobacter baumannii sequence type 345 of human and food origin from the Czech Republic, the first case in Europe. Antimicrobial Agents and Chemotherapy. 2019;63 (10):e01166-e01119. DOI: 10.1128/AAC.01166-19 - 57.
Martins-Sorenson N, Snesrud E, Xavier DE, Cacci LC, Iavarone AT, McGann P, et al. A novel plasmid-encoded mcr-4.3 gene in a colistin-resistant Acinetobacter baumannii clinical strain. The Journal of Antimicrobial Chemotherapy. 2020;75 (1):60-64. DOI: 10.1093/jac/dkz413 - 58.
Hindler JA, Humphries RM. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant gram-negative bacilli. Journal of Clinical Microbiology. 2013; 51 (6):1678-1684. DOI: 10.1128/JCM.03385-12 - 59.
Hong YK, Kim H, Ko KS. Two types of colistin heteroresistance in Acinetobacter baumannii isolates. Emerging Microbes & Infections. 2020;9 (1):2114-2123. DOI: 10.1080/22221751.2020.1821584 - 60.
Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii . Antimicrobial Agents and Chemotherapy. 2006;50 (9):2946-2950. DOI: 10.1128/AAC.00103-06 - 61.
Lo-Ten-Foe JR, de Smet AM, Diederen BM, Kluytmans JA, van Keulen PH. Comparative evaluation of the VITEK 2, disk diffusion, e-test, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae andAcinetobacter baumannii strains. Antimicrobial Agents and Chemotherapy. 2007;51 (10):3726-3730. DOI: 10.1128/AAC.01406-06 - 62.
Falagas ME, Makris GC, Dimopoulos G, Matthaiou DK. Heteroresistance: A concern of increasing clinical significance? Clinical Microbiology and Infection. 2008; 14 (2):101-104. DOI: 10.1111/j.1469-0691.2007.01912.x - 63.
Hawley JS, Murray CK, Jorgensen JH. Colistin heteroresistance in Acinetobacter and its association with previous colistin therapy. Antimicrobial Agents and Chemotherapy. 2008;52 (1):351-352. DOI: 10.1128/AAC.00766-07 - 64.
Chen L, Lin J, Lu H, Zhang X, Wang C, Liu H, et al. Deciphering colistin heteroresistance in Acinetobacter baumannii clinical isolates from Wenzhou, China. Journal of Antibiotics (Tokyo). 2020;73 (7):463-470. DOI: 10.1038/s4142 9-020-0289-2 - 65.
Karakonstantis S, Saridakis I. Colistin heteroresistance in Acinetobacter spp. : Systematic review and meta-analysis of the prevalence and discussion of the mechanisms and potential therapeutic implications. International Journal of Antimicrobial Agents. 2020;56 (2):106065. DOI: 10.1016/j.ijantimicag.2020.106065 - 66.
Rodriguez CH, De Ambrosio A, Bajuk M, Spinozzi M, Nastro M, Bombicino K, et al. In vitro antimicrobials activity against endemic Acinetobacter baumannii multiresistant clones. Journal of Infection in Developing Countries. 2010;4 (3):164-167. DOI: 10.3855/jidc.604 - 67.
Machado D, Antunes J, Simões A, Perdigão J, Couto I, McCusker M, et al. Contribution of efflux to colistin heteroresis tance in a multidrug-resistant Acinetobacter baumannii clinical isolate. Journal of Medical Microbiology. 2018;67 (6):740-749. DOI: 10.1099/jmm.0.000741 - 68.
Kon H, Hameir A, Temkin E, Keren-Paz A, Schwartz D, Schechner V, et al. Colistin dependency among colistin-heteroresistant Acinetobacter baumannii isolates. Microorganisms. 2021;10 (1):58. DOI: 10.3390/microorganis ms 10010058 - 69.
Lee JY, Chung ES, Ko KS. Transition of colistin dependence into colistin resistance in Acinetobacter baumannii . Scientific Reports. 2017;7 (1):14216. DOI: 10.1038/s415 98-017-14609-0 - 70.
Jovcic B, Novovic K, Dekic S, Hrenovic J. Colistin resistance in environmental isolates of Acinetobacter baumannii . Microbial Drug Resistance. 2021;27 (3):328-336. DOI: 10.1089/mdr.2020.0188 - 71.
Charretier Y, Diene SM, Baud D, Chatellier S, Santiago-Allexant E, van Belkum A, et al. Colistin heteroresistance and involvement of the PmrAB regulatory system in Acinetobacter baumannii . Antimicrobial Agents and Chemotherapy. 2018;62 (9):e00788-e00718. DOI: 10.1128/AAC.00788-18 - 72.
Yau W, Owen RJ, Poudyal A, Bell JM, Turnidge JD, Yu HH, et al. Colistin hetero-resistance in multidrug-resistant Acinetobacter baumannii clinical isolates from the Western Pacific region in the SENTRY antimicrobial surveillance program. The Journal of Infection. 2009;58 (2):138-144. DOI: 10.1016/j.jinf.2008.11.002 - 73.
Martínez-Martínez L. Muerte bacteriana y heterorresistencia a los antimicrobia nos [bacterial death and heteroresistance to antimicrobial agents]. Enfermedades Infecciosas y Microbiología Clínica. 2008; 26 (8):481-484. DOI: 10.1016/s0213-005x(08)72 774-5 - 74.
Li J, Rayner CR, Nation RL, Deans R, Boots R, Widdecombe N, et al. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrobial Agents and Chemotherapy. 2005; 49 (11):4814-4815. DOI: 10.1128/AAC.49.11.4814-4815.2005 - 75.
Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrobial Agents and Chemotherapy. 2009; 53 (8):3430-3436. DOI: 10.1128/AAC.01361-08 - 76.
Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrobial Agents and Chemotherapy. 2011; 55 (7):3284-3294. DOI: 10.1128/AAC.01733-10 - 77.
Karaiskos I, Friberg LE, Pontikis K, Ioannidis K, Tsagkari V, Galani L, et al. Colistin population pharmacokinetics after application of a loading dose of 9 MU colistin methanesulfonate in critically ill patients. Antimicrobial Agents and Chemotherapy. 2015; 59 (12):7240-7248. DOI: 10.1128/AAC.00554-15 - 78.
Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: Implications for selection of dosage regimens. Clinical Infectious Diseases. 2013; 57 (4):524-531. DOI: 10.1093/cid/cit 334 - 79.
Falagas ME, Kasiakou SK. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clinical Infectious Diseases. 2005; 40 (9):1333-1341. DOI: 10.1086/429323 - 80.
Fass RJ, Barnishan J, Ayers LW. Emergence of bacterial resistance to imipenem and ciprofloxacin in a university hospital. The Journal of Antimicrobial Chemotherapy. 1995; 36 (2):343-353. DOI: 10.1093/jac/36.2.343 - 81.
Levin AS, Mendes CM, Sinto SI, Sader HS, Scarpitta CR, Rodrigues E, et al. An outbreak of multiresistant Acinetobacter baumanii in a university hospital in São Paulo, Brazil. Infection Control and Hospital Epidemiology. 1996;17 (6):366-368. DOI: 10.1086/647319 - 82.
Dalla-Costa LM, Coelho JM, Souza HA, Castro ME, Stier CJ, Bragagnolo KL, et al. Outbreak of carbapenem-resistant Acinetobacter baumannii producing the OXA-23 enzyme in Curitiba, Brazil. Journal of Clinical Microbiology. 2003;41 (7):3403-3406. DOI: 10.1128/JCM.41.7.3403-34 06.2003 - 83.
Evans ME, Feola DJ, Rapp RP. Polymyxin B sulfate and colistin: Old antibiotics for emerging multiresistant gram-negative bacteria. The Annals of Pharmacotherapy. 1999; 33 (9):960-967. DOI: 10.1345/aph.18426 - 84.
Falagas ME, Kasiakou SK. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Critical Care. 2006; 10 (1):R27. DOI: 10.1186/cc3995 - 85.
Kunin CM, Bugg A. Binding of polymyxin antibiotics to tissues: The major determinant of distribution and persistence in the body. The Journal of Infectious Diseases. 1971; 124 (4):394-400. DOI: 10.1093/infdis/124.4.394 - 86.
Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Annals of Internal Medicine. 1970; 72 (6):857-868. DOI: 10.7326/0 003-4819-72-6-857 - 87.
Ledson MJ, Gallagher MJ, Cowperthwaite C, Convery RP, Walshaw MJ. Four years experience of intravenous colomycin in an adult cystic fibrosis unit. The European Respiratory Journal. 1998; 12 (3):592-594. DOI: 10.1183/09031936.98.12030592 - 88.
Price DJ, Graham DI. Effects of large doses of colistin sulphomethate sodium on renal function. British Medical Journal. 1970; 4 (5734):525-527. DOI: 10.1136/bmj.4.5734.5 25 - 89.
Tallgren LG, Liewendahl K, Kuhlbaeck B. The therapeutic success and nephrotoxicity of colistin in acute and chronic nephropathies with impaired renal function. Acta Medica Scandinavica. 1965; 177 :717-728. DOI: 10.1111/j.0954-6820.1965. tb01882.x - 90.
Abdelraouf K, He J, Ledesma KR, Hu M, Tam VH. Pharmacokinetics and renal disposition of polymyxin B in an animal model. Antimicrobial Agents and Chemotherapy. 2012; 56 (11):5724-5727. DOI: 10.1128/AAC.01333-12 - 91.
Akajagbor DS, Wilson SL, Shere-Wolfe KD, Dakum P, Charurat ME, Gilliam BL. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clinical Infectious Diseases. 2013; 57 (9):1300-1303. DOI: 10.1093/cid/cit453 - 92.
Phe K, Lee Y, McDaneld PM, Prasad N, Yin T, Figueroa DA, et al. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrobial Agents and Chemotherapy. 2014; 58 (5):2740-2746. DOI: 10.1128/AAC.024 76-13 - 93.
Nation RL, Rigatto MHP, Falci DR, Zavascki AP. Polymyxin acute kidney injury: Dosing and other strategies to reduce toxicity. Antibiotics (Basel). 2019; 8 (1):24. DOI: 10.3390/antibiotics8010024 - 94.
Zavascki AP, Nation RL. Nephrotoxicity of Polymyxins: Is there any difference between colistimethate and polymyxin B? Antimicrobial Agents and Chemotherapy. 2017; 61 (3):e02319-e02316. DOI: 10.1128/AAC.02319-16 - 95.
Deng Y, Gu JY, Li X, Tong H, Guo SW, Xu B, et al. Does monitoring total and free polymyxin b1 plasma concentrations predict polymyxin B-induced nephrotoxicity? A retrospective study in critically ill patients. Infectious Disease and Therapy. 2022; 11 (4):1591-1608. DOI: 10.1007/s40121-022-00655-3 - 96.
Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multiresistant gram-negative bacteria. International Journal of Antimicrobial Agents. 2005; 25 (1):11-25. DOI: 10.1016/j.ijantimicag.2004.10.001 - 97.
Pastewski AA, Caruso P, Parris AR, Dizon R, Kopec R, Sharma S, et al. Parenteral polymyxin B use in patients with multidrug-resistant gram-negative bacteremia and urinary tract infections: A retrospective case series. The Annals of Pharmacotherapy. 2008; 42 (9):1177-1187. DOI: 10.1345/aph.1K346 - 98.
Elias LS, Konzen D, Krebs JM, Zavascki AP. The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. The Journal of Antimicrobial Chemotherapy. 2010; 65 (10):2231-2237. DOI: 10.1093/jac/dkq285 - 99.
Kubin CJ, Ellman TM, Phadke V, Haynes LJ, Calfee DP, Yin MT. Incidence and predictors of acute kidney injury associated with intravenous polymyxin B therapy. The Journal of Infection. 2012; 65 (1):80-87. DOI: 10.1016/j.jinf.2012.01.015 - 100.
Mingeot-Leclercq MP, Tulkens PM, Denamur S, Vaara T, Vaara M. Novel polymyxin derivatives are less cytotoxic than polymyxin B to renal proximal tubular cells. Peptides. 2012; 35 (2):248-252. DOI: 10.1016/j.peptides.2012.03. 033 - 101.
Falagas ME, Kyriakidou M, Voulgaris GL, Vokos F, Politi S, Kechagias KS. Clinical use of intravenous polymyxin B for the treatment of patients with multidrug-resistant gram-negative bacterial infections: An evaluation of the current evidence. The Journal of Global Antimicrobial Resistance. 2021; 24 :342-359. DOI: 10.1016/j.jgar.202 0.12.026 - 102.
Aggarwal R, Dewan A. Comparison of nephrotoxicity of colistin with polymyxin B administered in currently recommended doses: A prospective study. Annals of Clinical Microbiology and Antimicrobials. 2018; 17 (1):15. DOI: 10.1186/s12941-018-0262-0 - 103.
Levin AS, Barone AA, Penço J, Santos MV, Marinho IS, Arruda EA, et al. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa andAcinetobacter baumannii . Clinical Infectious Diseases. 1999;28 (5):1008-1011. DOI: 10.1086/514732 - 104.
Garnacho-Montero J, Ortiz-Leyba C, Jiménez-Jiménez FJ, Barrero-Almodóvar AE, García-Garmendia JL, Bernabeu-WittelI M, et al. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: A comparison with imipenem-susceptible VAP. Clinical Infectious Diseases. 2003;36 (9):1111-1118. DOI: 10.1086/374337 - 105.
Ouderkirk JP, Nord JA, Turett GS, Kislak JW. Polymyxin B nephrotoxicity and efficacy against nosocomial infections caused by multiresistant gram-negative bacteria. Antimicrobial Agents and Chemotherapy. 2003; 47 (8):2659-2662. DOI: 10.1128/AAC.47.8.2659-2662.2003 - 106.
Markou N, Apostolakos H, Koumoudiou C, Athanasiou M, Koutsoukou A, Alamanos I, et al. Intravenous colistin in the treatment of sepsis from multiresistant gram-negative bacilli in critically ill patients. Critical Care. 2003; 7 (5):R78-R83. DOI: 10.1186/cc2358 - 107.
Sobieszczyk ME, Furuya EY, Hay CM, Pancholi P, Della-Latta P, Hammer SM, et al. Combination therapy with polymyxin B for the treatment of multidrug-resistant gram-negative respiratory tract infections. The Journal of Antimicrobial Chemotherapy. 2004; 54 (2):566-569. DOI: 10.1093/jac/dkh369 - 108.
Falagas ME, Rizos M, Bliziotis IA, Rellos K, Kasiakou SK, Michalopoulos A. Toxicity after prolonged (more than four weeks) administration of intravenous colistin. BMC Infectious Diseases. 2005; 5 :1. DOI: 10.1186/1471-2334-5-1 - 109.
Reina R, Estenssoro E, Sáenz G, Canales HS, Gonzalvo R, Vidal G, et al. Safety and efficacy of colistin in Acinetobacter andPseudomonas infections: A prospective cohort study. Intensive Care Medicine. 2005;31 (8):1058-1065. DOI: 10.1007/s00134-005-2691-4 - 110.
Michalopoulos AS, Tsiodras S, Rellos K, Mentzelopoulos S, Falagas ME. Colistin treatment in patients with ICU-acquired infections caused by multiresistant gram-negative bacteria: The renaissance of an old antibiotic. Clinical Microbiology and Infection. 2005; 11 (2):115-121. DOI: 10.1111/j.1469-0691.2004.01043.x - 111.
Kasiakou SK, Michalopoulos A, Soteriades ES, Samonis G, Sermaides GJ, Falagas ME. Combination therapy with intravenous colistin for management of infections due to multidrug-resistant gram-negative bacteria in patients without cystic fibrosis. Antimicrobial Agents and Chemotherapy. 2005; 49 (8):3136-3146. DOI: 10.1128/AAC.49.8.3136-3146.2005 - 112.
Holloway KP, Rouphael NG, Wells JB, King MD, Blumberg HM. Polymyxin B and doxycycline use in patients with multidrug-resistant Acinetobacter baumannii infections in the intensive care unit. The Annals of Pharmacotherapy. 2006;40 (11):1939-1945. DOI: 10.1345/aph.1H353 - 113.
Ramasubban S, Majumdar A, Das PS. Safety and efficacy of polymyxin B in multidrug-resistant gram-negative severe sepsis and septic shock. Indian Journal of Critical Care Medicine. 2008; 12 (4):153-157. DOI: 10.4103/0972-5229.45074 - 114.
Oliveira MS, Prado GV, Costa SF, Grinbaum RS, Levin AS. Polymyxin B and colistimethate are comparable as to efficacy and renal toxicity. Diagnostic Microbiology and Infectious Disease. 2009; 65 (4):431-434. DOI: 10.1016/j.diagmicrobio.2009.07.018 - 115.
Mendes CA, Cordeiro JA, Burdmann EA. Prevalence and risk factors for acute kidney injury associated with parenteral polymyxin B use. The Annals of Pharmacotherapy. 2009; 43 (12):1948-1955. DOI: 10.1345/aph.1M277 - 116.
Esaian D, Dubrovskaya Y, Phillips M, Papadopoulos J. Effectiveness and tolerability of a polymyxin B dosing protocol. The Annals of Pharmacotherapy. 2012; 46 (3):455-456. DOI: 10.1345/aph.1Q294 - 117.
Nandha R, Sekhri K, Mandal AK. To study the clinical efficacy and nephrotoxicity along with the risk factors for acute kidney injury associated with parenteral polymyxin B. Indian Journal of Critical Care Medicine. 2013; 17 (5):283-287. DOI: 10.4103/0972-5229.120319 - 118.
Crusio R, Rao S, Changawala N, Paul V, Tiu C, van Ginkel J, et al. Epidemiology and outcome of infections with carbapenem-resistant gram-negative bacteria treated with polymyxin B-based combination therapy. Scandinavian Journal of Infectious Diseases. 2014; 46 (1):1-8. DOI: 10.3109/00365548.2013.844350 - 119.
Tuon FF, Rigatto MH, Lopes CK, Kamei LK, Rocha JL, Zavascki AP. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. International Journal of Antimicrobial Agents. 2014; 43 (4):349-352. DOI: 10.1016/j.ijantimicag.2013.12.002 - 120.
Siddiqui NU, Qamar FN, Jurair H, Haque A. Multidrug-resistant gram-negative infections and use of intravenous polymyxin B in critically ill children of a developing country: Retrospective cohort study. BMC Infectious Diseases. 2014; 14 :626. DOI: 10.1186/s12879-014-0626-9 - 121.
Rigatto MH, Behle TF, Falci DR, Freitas T, Lopes NT, Nunes M, et al. Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: A multicentre prospective cohort study. The Journal of Antimicrobial Chemotherapy. 2015; 70 (5):1552-1557. DOI: 10.1093/jac/dku561 - 122.
Nelson BC, Eiras DP, Gomez-Simmonds A, Loo AS, Satlin MJ. Jenkins SGet al. Clinical outcomes associated with polymyxin B dose in patients with bloodstream infections due to carbapenem-resistant gram-negative rods. Antimicrobial Agents and Chemotherapy. 2015; 59 (11):7000-7006. DOI: 10.1128/AAC.00844-15 - 123.
Dubrovskaya Y, Prasad N, Lee Y, Esaian D, Figueroa DA, Tam VH. Risk factors for nephrotoxicity onset associated with polymyxin B therapy. The Journal of Antimicrobial Chemotherapy. 2015; 70 (6):1903-1907. DOI: 10.1093/jac/dkv014 - 124.
Rigatto MH, Oliveira MS, Perdigão-Neto LV, Levin AS, Carrilho CM, Tanita MT, et al. Multicenter prospective cohort study of renal failure in patients treated with colistin versus polymyxin B. Antimicrobial Agents and Chemotherapy. 2016; 60 (4):2443-2449. DOI: 10.1128/AAC. 02634-15 - 125.
Cai B, Cai Y, Liew YX, Chua NG, Teo JQ , Lim TP, et al. Clinical efficacy of polymyxin monotherapy versus nonvalidated polymyxin combination therapy versus validated polymyxin combination therapy in extensively drug-resistant gram-negative bacillus infections. Antimicrobial Agents and Chemotherapy. 2016; 60 (7):4013-4022. DOI: 10.1128/AAC.03 064-15 - 126.
Ismail B, Shafei MN, Harun A, Ali S, Omar M, Deris ZZ. Predictors of polymyxin B treatment failure in gram-negative healthcare-associated infections among critically ill patients. Journal of Microbiology, Immunology, and Infection. 2018; 51 (6):763-769. DOI: 10.1016/j.jmii.2017.03.007 - 127.
John JF, Falci DR, Rigatto MH, Oliveira RD, Kremer TG, Zavascki AP. Severe infusion-related adverse events and renal failure in patients receiving high-dose intravenous polymyxin B. Antimicrobial Agents and Chemotherapy. 2017; 62 (1):e01617-e01617. DOI: 10.1128/AAC.01617-17 - 128.
Okoduwa A, Ahmed N, Guo Y, Scipione MR, Papadopoulos J, Eiras DP, et al. Nephrotoxicity associated with intravenous polymyxin B once- versus twice-daily dosing regimen. Antimicrobial Agents and Chemotherapy. 2018; 62 (8):e00025-e00018. DOI: 10.1128/AAC.00025-18 - 129.
Zheng JY, Huang SS, Huang SH, Ye JJ. Colistin for pneumonia involving multidrug-resistant Acinetobacter calcoaceticus-Acinetobacter baumannii complex. Journal of Microbiology, Immunology, and Infection. 2020;53 (6):854-865. DOI: 10.1016/j.jmii.2019. 08.007 - 130.
Li KL, Abad CLR. The clinical profile and outcomes of adult patients given intravenous colistin for multidrug-resistant gram-negative infections in a Philippine tertiary hospital. International Journal of Infectious Diseases. 2020; 93 :9-14. DOI: 10.1016/j.ijid. 2020.01.022 - 131.
Kagami K, Ishiguro N, Yamada T, Niinuma Y, Iwasaki S, Taki K, et al. Efficacy and safety of colistin for the treatment of infections caused by multidrug-resistant gram-negative bacilli. Journal of Infection and Chemotherapy. 2021; 27 (3):473-479. DOI: 10.1016/j.jiac.2020.10.024 - 132.
Aitullina A, Purviņa S, Krūmiņa A. Colistin co-administration with other nephrotoxins: Experience of teaching hospital of Latvia. International Journal of Clinical Pharmacy. 2021; 43 (3):509-517. DOI: 10.1007/s11096-020-01154-6 - 133.
Saelim W, Changpradub D, Thunyaharn S, Juntanawiwat P, Nulsopapon P, Santimaleeworagun W. Colistin plus sulbactam or fosfomycin against carbape nem-resistant Acinetobacter baumannii : Improved efficacy or decreased risk of nephrotoxicity? Infection & Chemotherapy. 2021;53 (1):128-140. DOI: 10.3947/ic.202 1.0007 - 134.
Jeong YJ, Gu N, Kwack WG, Kang Y, Park SY, Yoon YS. Prospective observational study of the impact of plasma colistin levels in patients with carbapenem-resistant Acinetobacter baumannii pneumonia. The Journal of Global Antimicrobial Resistance. 2021;27 :315-323. DOI: 10.1016/j.jgar.2021.10.017 - 135.
Sadyrbaeva-Dolgova S, García-Fumero R, Exposito-Ruiz M, Pasquau-Liaño J, Jiménez-Morales A, Hidalgo-Tenorio C. Incidence of nephrotoxicity associated with intravenous colistimethate sodium administration for the treatment of multidrug-resistant gram-negative bacterial infections. Scientific Reports. 2022; 12 (1):15261. DOI: 10.1038/s41598-022-19626-2 - 136.
Keski NAS, Seyman D, Önder KD, Kizilateş F, Keski NO. Investigation of effect of the colistin loading dosage on the clinical, microbiological, and laboratory results in Acinetobacter baumannii ventilator-associated pneumonia/pneumonia. International Journal of Clinical Practice. 2022;2022 :5437850. DOI: 10.1155/2022/5437850 - 137.
Koza Y. Acute kidney injury: Current concepts and new insights. Journal of Injury and Violence Research. 2016; 8 (1):58-62. DOI: 10.5249/jivr.v8i1.610 - 138.
Wasung ME, Chawla LS, Madero M. Biomarkers of renal function, which and when? Clinica Chimica Acta. 2015; 438 :350-357. DOI: 10.1016/j.cca.2014.08.039 - 139.
Wolinsky E, Hines JD. Neurotoxic and nephrotoxic effects of colistin in patients with renal disease. The New England Journal of Medicine. 1962; 266 :759-762. DOI: 10.1056/NEJM 196204122661505 - 140.
Lindesmith LA, Baines RD Jr, Bigelow DB, Petty TL. Reversible respiratory paralysis associated with polymyxin therapy. Annals of Internal Medicine. 1968; 68 (2):318-327. DOI: 10.7326/0003-4819-68-2-318 - 141.
Cox CE, Harrison LH. Intravenous sodium colistimethate therapy of urinary tract infections: Pharmacological and bacteriological studies. Antimicrobial Agents and Chemotherapy (Bethesda). 1970; 10 :296-302 - 142.
Fekety FR Jr, Norman PS, Cluff LE. The treatment of gram-negative bacillary infections with colistin. The toxicity and efficacy of large doses in forty-eight patients. Annals of Internal Medicine. 1962; 57 :214-229. DOI: 10.7326/0003-4819-57-2-214 - 143.
Hopper J Jr, Jawetz E, Hinman F Jr. Polymyxin B in chronic pyelonephritis: Observations on the safety of the drug and on its influence on the renal infection. The American Journal of the Medical Sciences. 1953; 225 (4):402-409 - 144.
Mcmillan M, Price TM, Maclaren DM, Scott GW. Pseudomonas pyocyanea infection treated with colistin methane sulphonate. Lancet. 1962;2 (7259):737-739. DOI: 10.1016/s0140-6736(62)90568-8 - 145.
Dai C, Ciccotosto GD, Cappai R, Tang S, Li D, Xie S, et al. Curcumin attenuates colistin-induced neurotoxicity in N2a cells via anti-inflammatory activity, suppression of oxidative stress, and apoptosis. Molecular Neurobiology. 2018; 55 (1):421-434. DOI: 10.1007/s12035-016-0276-6 - 146.
Dai C, Li J, Lin W, Li G, Sun M, Wang F, et al. Electrophysiology and ultrastructural changes in mouse sciatic nerve associated with colistin sulfate exposure. Toxicology Mechanisms and Methods. 2012; 22 (8):592-596. DOI: 10.3109/15376516.2012.704956 - 147.
Dai C, Li J, Li J. New insight in colistin induced neurotoxicity with the mitochondrial dysfunction in mice central nervous tissues. Experimental and Toxicologic Pathology. 2013; 65 (6):941-948. DOI: 10.1016/j.etp.2013.01.008 - 148.
Dai C, Tang S, Velkov T, Xiao X. Colistin-induced apoptosis of neuroblastoma-2a cells involves the generation of reactive oxygen species, mitochondrial dysfunction, and autophagy. Molecular Neurobiology. 2016; 53 (7):4685-4700. DOI: 10.1007/s12035-015-9396-7 - 149.
Dai C, Ciccotosto GD, Cappai R, Wang Y, Tang S, Xiao X, et al. Minocycline attenuates colistin-induced neurotoxicity via suppression of apoptosis, mitochondrial dysfunction, and oxidative stress. The Journal of Antimicrobial Chemotherapy. 2017; 72 (6):1635-1645. DOI: 10.1093/jac/dkx037 - 150.
Mattos KPH, Cintra ML, Gouvêa IR, Ferreira LÁ, Velho PENF, Moriel P. Skin hyperpigmentation following intravenous polymyxin B treatment associated with melanocyte activation and inflammatory process. Journal of Clinical Pharmacy and Therapeutics. 2017; 42 (5):573-578. DOI: 10.1111/jcpt.12543 - 151.
Knueppel RC, Rahimian J. Diffuse cutaneous hyperpigmentation due to tigecycline or polymyxin B. Clinical Infectious Diseases. 2007; 45 (1):136-138. DOI: 10.1086/518706 - 152.
Mattos KP, Lloret GR, Cintra ML, Gouvêa IR, Betoni TR, Mazzola PG, et al. Acquired skin hyperpigmentation following intravenous polymyxin B treatment: A cohort study. Pigment Cell & Melanoma Research. 2016; 29 (3):388-390. DOI: 10.1111/pcmr.12468 - 153.
Gothwal S, Meena K, Sharma SD. Polymyxin B induced generalized hyperpigmentation in neonates. Indian Journal of Pediatrics. 2016; 83 (2):179-180. DOI: 10.1007/s12098-015-1798-z - 154.
Zavascki AP, Manfro RC, Maciel RA, Falci DR. Head, and neck hyperpigmentation probably associated with polymyxin B therapy. The Annals of Pharmacotherapy. 2015; 49 (10):1171-1172. DOI: 10.1177/1060028015595643 - 155.
Lahiry S, Choudhury S, Mukherjee A, Bhunya PK, Bala M. Polymyxin B-induced diffuse cutaneous hyperpigmentation. Journal of Clinical and Diagnostic Research. 2017; 11 (2):FD01-FD02. DOI: 10.7860/JCDR/2017/24278.9213 - 156.
Zavascki AP, Schuster LF, Duquia RP. Histopathological findings of pigmented lesion and recovery of natural skin color in a patient with polymyxin B-associated diffuse hyperpigmentation. International Journal of Antimicrobial Agents. 2016; 48 (5):579-580. DOI: 10.1016/j.ijantimicag.2016.08.010 - 157.
Zheng G, Cao L, Che Z, Mao E, Chen E, He J. Polymyxin B-induced skin hyperpig mentation: A rare case report and literature review. BMC Pharmacology and Toxicology. 2018; 19 (1):41. DOI: 10.1186/s40360-018-0226-1 - 158.
Li YM, Milikowski C, Selvaggi G, Abbo LM, Skiada D, Galimberti F. Polymyxin B-induced skin hyperpigmentation. Transplant Infectious Disease. 2020; 22 (5):e13312. DOI: 10.1111/tid.13312 - 159.
Silpa-Archa N, Kohli I, Chaowattanapanit S, Lim HW, Hamzavi I. Postinflammatory hyperpigmentation: A comprehensive overview: Epidemiology, pathogenesis, clinical presentation, and noninvasive assessment technique. Journal of the American Academy of Dermatology. 2017; 77 (4):591-605. DOI: 10.1016/j.jaad.2017.01.035 - 160.
Lu C, Hou N. Skin hyperpigmentation in coronavirus disease 2019 patients: Is polymyxin B the culprit? Frontiers in Pharmacology. 2020; 11 :01304. DOI: 10.3389/fphar.2020.01304