Stress memory in various plant species.
Abstract
Humankind interfered in the natural selection of plants in favor of traits such as yield, grain quality, productivity, and flavor principally at the expense of several biotic and abiotic stress tolerance capacities. Plants are subjected to the detrimental effects of the combination of these factors due to their stationary nature. Today, there are various breeding approaches from classical to transgenesis and even genome editing to tame plant genome for our purposes. Additionally, the significance of epigenetic regulation in response to biotic and abiotic stresses has been recognized in the last decade. Acquisition and preservation of stress memory for the progeny to allow them to adapt to similar conditions through methylation, histone modification, and chromatin structure alterations are the focus of attention. Enlightening the cross talk between these components of acquired transgenerational memory may aid to breed more efficient and environmentally friendly crops in current agricultural systems. Priming applications have been extensively studied to induce stress memory of the plant by external stimulus as a warning signal, which may ignite minor activations of stress-responsive gene expression and eventually turn into strong resistance. The present chapter will discuss the basis and the recent advances in plant epigenetic regulation with emphasis on chemical, biotic, and abiotic priming agents.
Keywords
- epigenetics
- epigenomics
- histone modifications
- methylation
- plant breeding
1. Introduction
Crop production and global food security are endangered due to the rapid increase in the world population and the continuous emergence of drought, flood, heat wave, and frost events along with other global climate change-related issues. Therefore, new plant breeding strategies are required for achieving stress tolerance, increasing yield, improving crop quality, and creating more adaptive and sustainable germplasms for future climate challenges. In recent years, transgenic technology and genome editing techniques, which include the transformation and genetic modification of single or multiple genes, as well as classical or mutation breeding, have been used efficiently for stress-tolerant or -resistant plant breeding. Clearly, all breeding methods have advantages and disadvantages for the improvement of agricultural traits. Classical breeding and hybridization are the most common and traditional methods for environmental stress-tolerant varieties. However, the breeding process depends on the availability of the germplasm that has the desired traits. The breeding process takes a very long time throughout generations. Traits are only inherited between closely related species. Selection of the desired traits in candidate lines requires intensive labor. All these limitations led plant breeders to search for new strategies as alternatives to classical breeding. In mutation breeding, various physical, chemical, or biological mutagens are used to increase genetic variability. High mutation frequency is obtained compared to naturally occurring spontaneous mutations [1, 2, 3, 4]. The selection process requirement in each generation is a major disadvantage of mutation breeding. On the other hand, transgenic technology has revolutionized as the fastest adopted crop technology in the history of modern agriculture, which enables the improvement of plants with the predictable changes for target trait in a relatively short time [5, 6, 7, 8]. However, the restrictive effects of legal regulations regarding the cultivation of genetically modified crops and their transport into other countries are some of the obstacles in front of this strategy as well as metabolic imbalance or off-target effects caused by genetic modification [9, 10]. All these difficulties encountered in various plant breeding strategies and the radical expansion of the genetic and epigenetic information pool have paved the way for the testing of novel strategies.
Since plants have a stationary lifestyle and lack adaptive immune system as well as specific immune response cells, they require highly sophisticated regulatory mechanisms for defense. Memorizing a past stressor and the proper response is extremely cost-effective to plants compared to constitutive preparedness. Since plants lack the nervous system and a central brain, retrieving the previous response to a specific stressor after a period of unstressed condition may delay effective counteract. In this regard, various priming applications are getting tested today to put plants into a physiological state that allows them to respond more rapidly and/or more robustly after exposure to a particular biotic or abiotic stress. In this state, plants have enhanced stress perception due to the amplified stress signaling that leads to more efficient activation of the defense response and enhanced resistance before encountering the stressor, instead of stress response-related gene induction [11]. Stress-induced memory is mediated by metabolomic (amino acids, sugars, etc.), proteomic (antioxidant and photosynthesis enzymes etc.), transcriptomic (WRKY, AREB, etc.), epigenetic (DNA methylation, chromatin remodeling, etc.) mechanisms. These mechanisms can be transferred to future generations [12]. Among these mechanisms, epigenetics particularly play an important role in creating, preserving, and transmitting stress memory to the next generations. Some stress-induced epigenetic changes can act as a transmittable memory for the progeny. For these reasons, it is important to comprehend three main mechanisms of epigenetic regulation to understand stress memory and the basis of priming applications: DNA methylation, histone modifications, and RNA-mediated gene silencing.
In the last decade, epigenetic regulatory mechanism of gene function without alteration of the DNA sequence was an intense research topic. In 1994, Wassenegger and colleagues discovered induction of
Histones are the central components of chromatin organization. There is a group of histones that are predominantly dependent on DNA replication known as canonical histones and also a group called replacement histones that are expressed all along the cell cycle and independent of DNA replication. They are localized in specific regions in the genome and have their unique sequences among themselves. Replacement histones, also known as histone variants, are found in all studied model organisms. Some of them can be lineage-, tissue-, or domain-specific. For instance, H2A.X and H2A.Z are evolutionarily conserved from yeast to mammals, whereas macroH2A and H2A.Bbd are found only in mammals and H2A.W is specific to plants [16]. Eukaryotic genomes consist of repeating nucleosomes that are octamer histones wrapped around 147 base pairs of DNA. Nucleosomes that were previously believed to provide a universal, nonspecific coating of genomic DNA are well-known to have favored positions throughout the genome [17]. Depending on the nucleosome composition, chromatin either can be more accessible to transcription or has closed conformation. The different states of chromatin are constituted from diverse canonical and replacement histone combinations. H3.3, H2A.Z, and H2A.X variants are abundant in euchromatic regions. H3.3 evolved independently in plants and animals. The evidence of convergent evolution strongly points toward the importance of H3.3 to the function of the eukaryotic genome [18]. H2A.Z which has a single evolutionary origin nearly in branches of Eukarya. It plays pivotal roles in multicellular development. It has been linked to various biological (plant immunity, germline development, and stress response) and cellular (genome stability and DNA repair) processes as well as both transcriptional activation and repression. H2A.X is found in most of the eukaryotes; however, it has evolved multiple times contrary to the H2A.Z. Phosphorylated H2A.X is suggested to be a histone mark for DNA damage repair (DDR) [18]. There are other histone marks in chromatin state typical of active transcription. H3K4me3 is particularly enriched at the transcriptional start site of actively transcribed genes. H3K36me3 differs from that in animals as it marks active genes in plants at 5′ region similar to H3K4me3. It is related to gene body of active genes. H2B can be monoubiquitinated at their C-terminal residues (H2Bub1). This mark is usually related to active genes and associated to genomic regions in both H3K4me3 and H3K36me3 [19]. On the other hand, there are also heterochromatin marks in silent genomic regions allowing chromatin compaction such as H3K9me2, H3K27me3, H3K27me1 [20]. During stress conditions, some histone variants are incorporated in the nucleosomes of stress-responsive genes. They may have various posttranscriptional modifications (PTMs) such as acetylation, methylation, and phosphorylation. The histone marks and their PTMs allow a wide variety of nucleosome combinations. Therefore, they are responsible for storing plant epigenetic memory under different circumstances [21].
Small RNAs (such as micro RNAs and small interfering RNAs) play an important role in regulating gene expression through transcriptional gene silencing (TGS) or posttranscriptional gene silencing (PTGS) under environmental stress. Stress-induced synthesis of small RNAs can reduce the expression of genes that would be affected by stress conditions. Conversely, they can downregulate their own expression to increase the expression of target genes. There have been many studies showing the relationship between stress memory and small RNAs [22, 23]. The chromatin remodeling machinery, which may act alone or in complexes, is controlled by CHR (chromatin remodeling factors) proteins such as histone chaperones, histone-modifying enzymes, and ATP-dependent chromatin remodeling complexes. Chromatin remodeling compresses and unfolds nucleosome DNA. Therefore, DNA becomes accessible for replication, repair, and selective gene expression. In this way, it plays a role as an important mechanism in the biotic and/or abiotic stress response in plants. Numerous evidence presented the relationship between stress memory and chromatin remodeling [24, 25, 26].
The existence of epigenetic memory within the generation is called intragenerational adaptation. Most of the epigenetic alterations are reversible after prolonged unstressed conditions; however, subsequent exposures may lead to stress training. It has been well-documented particularly for drought stress memory [27, 28]. It is also known that some of these trained chromatin alterations are heritable by meiosis. They are called transgenerational inheritance of stress memory. They can be detected at least after a non-stressed generation of a parental plant that has experienced the environmental effect. Otherwise, if subsequent progeny does not present the trained responses, it is simply intragenerational adaptive phenotypic change in parents.
2. Stress memory in plants
Stress is a state where environmental conditions change enough to affect the normal growth and development of a plant adversely. Therefore, it leads to a wide range of molecular alterations throughout the organism. These effects can occur through living organisms (biotic) or environmental factors (abiotic). These stresses may occur unexpectedly or in a cyclical manner over days, seasons, or even years. Some stresses rarely occur throughout a plant’s life, but abiotic stresses such as temperature, drought, salinity, and cold are often repetitive. Repeated exposure to different types of stress and re-exposure to a previous stress can alter the biochemical, physiological, transcriptomic, proteomic [29], and metabolomic responses to subsequent stress. Unlike animals, plants are immobile organisms that do not have a specialized nervous system to store the “memories” of past stress events. Stress memory is defined as the capacity of plants to store this information in order to respond to recurring stress in the most rapid manner [30].
The term of “stress memory” in plants was first introduced in 90s, when it was observed that some plants developed resistance to subsequent pathogen attacks after being exposed to a previous pathogen attack [31]. Subsequently, advanced studies have shown that plants exhibit stronger responses to recurring stresses and display much higher tolerance capacities compared to those that have not previously encountered the stress [32].
The concept of stress memory has been categorized under three definitions by Lämke and Bäurle [33]. Somatic stress memory is observed throughout the lifetime of the organism exposed to stress and is mitotically inherited. Somatic stress memory is controlled through mechanisms such as DNA methylation, histone tail modifications, RNA polymerase II pausing, and the production of stress-related metabolites. Even after the stress factor is removed, mechanisms at the DNA, RNA, protein, and chromosome levels, such as gene expression, protein accumulation, and chromatin modifications, remain active [34]. Transgenerational memory (TSM) can be observed even after at least two stress-free generations and is meiotically inherited. It is based on epigenetic foundations. TSM is a phenomenon that surpasses both the offspring’s genotype and the direct environmental conditions’ impact, due to the transfer of information from stressed parents. TSM can involve the transmission of structural variations in the genome [35], the inheritance of chromatin modifications, or the storage of molecules as maternal-mediated mRNA, hormones, proteins, starch, lipids, and other compounds. Intergenerational memory (ISM) is observed in the first generation of offspring where stress was not encountered. ISM is a memory effect that can only be observed in the first generation where stress is absent. This phenomenon is mediated through the conditions of seed growth and signals produced by the parent plants in the seeds or embryos. The stress factor experienced in the previous generation also affects the reproductive cells and, consequently, the resulting seeds. Thanks to this effect, offspring exhibits phenotypes that cannot otherwise be explained, genotypically [36]. By studying the preconditioning of
| Plant species | Priming | Stress | Stress memory | Determination of stress memory | Ref. |
|---|---|---|---|---|---|
| S-methyl | Somatic | Histone methylation level measurement by chromatin immunoprecipitation | [37] | ||
| Heat 37°C | Heat 44°C | Somatic | Immunoprecipitation and mass spectrometry | [38] | |
| Salinity | Salinity | Intergenerational | DNA methylation level measurement by bisulfite sequencing | [39] | |
| β-Aminobutyric acid (BABA) | Intergenerational | DNA methylation level measurement by 5mC ELISA | [40] | ||
| Heat 30°C Cold 16°C | Heat 30°C Cold 16°C | Transgenerational | The number of flowers per plant | [41] | |
| Drought | Drought | Transgenerational | Measurements of photosynthesis and leaf conductance | [42] | |
| Drought | Drought | Transgenerational | Seed oil content, seed protein content, and seed metabolite content | [43] |
Table 1.
Molecular mechanisms related to stress memory are categorized into two main types as cis and trans. The term “cis” refers to the effects of a molecule or genetic material on itself, meaning that cis memory describes changes that occur within the same DNA molecule or genetic region. Cis memory mechanisms are directly influenced by physical markers such as DNA methylation or modifications to histone tails. On the other hand, “trans” memory refers to the effects of a molecule or genetic material on another gene region or molecule. Trans memory involves interactions that are controlled by feedback loops and transmitted to the offspring through cytoplasmic division. It is often used in prokaryotes and single-celled eukaryotes. By another definition, cis memory describes genetic changes that occur within the same gene or DNA region, while trans memory pertains to interactions between different regions of genetic material or molecules. These terms are used to explain processes of genetic-level information storage, transmission, and regulation [34].
Epigenetic mechanisms, including DNA methylation, modification of histone tails, noncoding RNAs, and suppression of 5-serine phosphorylation of RNA polymerase II, play a role in the formation of stress memory. However, besides the epigenetic mechanisms, other mechanisms based on the accumulation of dephosphorylated stress proteins activated by protein kinases, the production of signaling molecules involved in stress perception and tolerance, and changing the levels of transcription factors are also effective. The metabolite accumulation mechanism, which is more temporary, causes short-term stress effects in plants, unlike the long-term stress effects caused by epigenetic mechanisms [44].
Ali et al. reported that the effects of herbivore-induced plant volatiles (HIPV) were memorized and stored by plants. The plant recalls this memory later under secondary herbivore attacks. This epigenetically regulated memory is preserved for at least 5 days after exposure to HIPVs. Moreover, it is reported that serious methylation losses occurred in the promoter region of the Bowman-Birk-type trypsin inhibitor (TI) gene in
Priming contributes to stronger reactions and long-term survival against stress. This is very important to protect vital activities of the plants. Nevertheless, memory can also prevent the plant’s optimal development process. It requires intense energy to keep short-term (physiological, metabolic changes) and long-term (epigenetic) mechanisms ready for stress. Remaining in alerted state even under unstressed conditions may cause disruption of processes such as plant development and reproduction or increase sensitivity to harmful effects. Therefore, removing changes related to stress memory, especially in variable and unpredictable conditions, provides an advantage to the plant. The balance between this state and memory is called “memory resetting”. Resetting is established by RNA metabolism, posttranscriptional gene silencing, or RNA-directed DNA methylation [47, 48]. DNA methylation in eukaryotic genomes occurs by the addition of a methyl group to the 5th position of the pyrimidine ring of the cytosine base. While cytosine methylation can occur in the CG regions in other eukaryotes, cytosine methylation can occur in the CG, CHG, and CHH regions in plant genomes. This difference provides greater epigenetic diversity to plants. Another necessity of memory resetting is due to the density of these methylation regions. As a result of exposure to different stresses, cytosine methylation accumulates at a high rate because of memory formation. The accumulation of methylated cytosine density causes frequent cytosine-thymine exchange that may lead to the enhanced mutation frequency, if not reset [49].
Epigenetic variations in DNA and chromatin are extremely important for the formation of heterochromatin and euchromatin structures. DNA is kept in euchromatin state to respond to different exposed stress factors. This state maintains that the structure highly accessible to physical and chemical mutagens. However, DNA in heterochromatin structure is more protected from mutations. In this regard, euchromatinized DNA regions must be reduced by resetting to protect genome from mutations. Similarly, stress memory stored in the form of proteins must be reset over time. Cytosolic accumulation of proteins affects the functioning of cellular activities. Therefore, protein degradation often occurs through 26S proteasome or autophagy-related ubiquitination [50, 51].
Epigenetic changes that occur randomly or as a result of cellular stress can be inherited across successive generations if not corrected. After stress-induced DNA damage and the random loss of methylation in actively transcribing alleles after DNA damage, the replacement of methylated cytosines with unmethylated cytosines occurs through DNA repair mechanisms. The accumulation of these erroneous epigenetic marks across generations poses a significant problem for the organism. Epigenetic reprogramming mechanisms encompass chromatin remodeling factors and small noncoding RNAs (sncRNAs). They function during gametogenesis and early embryo development to prevent the transmission of acquired chromatin states.
In
3. Priming applications
The term priming was first introduced in biotic stress studies to ensure immunity against pathogens [53]. Priming involves pre-exposure of plant to an abiotic or biotic stressor to develop resistance. It can be applied at any developmental stage to improve stress tolerance. In addition to natural stressors, it can also be achieved through the application of synthetic stimulants that activate the plant defense systems [54, 55]. DNA methylation level alterations, histone tail modifications, transcription factors, and inactive mitogen-activated protein kinase accumulation are some important mechanisms of stress memory. These mechanisms are responsible for the re-emergence of stress responses in a much stronger and shorter period in the event of reoccurrence of stress [56].
Priming efficiency in inducing stress memory may vary depending on the preferred priming agent, application time, and plant species (Table 2). Therefore, a specific and optimized priming technique is required for each plant species. This is the primary disadvantage of priming, because it requires an extensive number of tests to determine the optimal dose and time for each plant type. There is also a need to develop storage strategies for preservation of primed seeds [64, 65, 66]. Optimization involves various parameters such as time required for treatment, priming or coating agent, seed viability, and storage conditions (temperature, humidity, oxygen requirement, etc.), which are standardized for each variety by trial [67]. Priming applications are usually carried out in two different ways for the formation of stress memory. They are seed priming and plant priming. It is an impressive and promising strategy to mitigate the impact of climate change on crops and improve agricultural traits, although still extensive work is required before its application to breeding programs in the field (Figure 1) [12, 68].
| Priming agent | Agent dose | Plant | Stress factor | Effect | Ref. |
|---|---|---|---|---|---|
| JA or SA | 0.1 mM 0.5 mM | (PstDC3000) | General DNA hypomethylation changes. Enrichment of H3K9ac and H3K27me3 in WRKY6 and WRKY53 genes | [57] | |
| 2,6-dichloroisonicotinic acid (INA) | 100 μM | INA-primed common bean plants and its stress-free offsprings exhibited enrichment of H3K4me3 and H3K36me3 (transgenerational immune memory) | [58] | ||
| β-Aminobutyric acid (BABA) | 5 mM | Primed tomato leaves close to 50% reduction in the frequency of mCHH in the genome, and while the net reduction in CG and CHG methylation reflected a balance of both hyper- and hypomethylation, almost all CHH DMRs (differentially methylated regions) were hypomethylated. | [59] | ||
| β-Aminobutyric acid (BABA) | By spraying potato leaves with 5 mM of BABA (3 ml per plant) | High levels of the heritable H3K4me2 tag in BABA primed seeds (F0) and their vegetative, productive progeny (F1) provided evidence for the epigenetic marker for intergenerational memory in potato | [60] | ||
| NaCI | 50 mM NaCl | Drought | Decrease in H3K27me3 at the chromatin level | [61] | |
| NaCl | 50 mM NaCl | Salinity stress | Increased expression of the Na-transporter HKT1 gene | [61] | |
| NaCI | % 0.3 NaCI | Salinity stress | H3K4me2, H3K4me3, and H3K9ac were dramatically induced | [62] | |
| Heavy metals | Cu2+ (1000 μM CuSO4), Cd2+ (1000 μM CdCl2), Cr3+ (1000 μM CrCl3) or Hg2+ (50 μM HgCl2) | Heavy metal | Changes associated with DNA methylation state of Tos17 retrotransposon | [63] |
Table 2.
Epigenetic effects of priming agent on plant species.
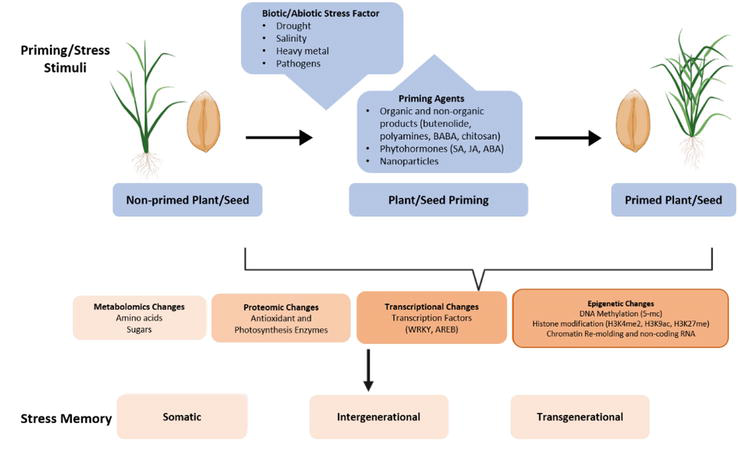
Figure 1.
Schematic representation of establishing stress memory through priming applications.
3.1 Seed priming
Prior to germination, seeds are treated with natural and synthetic components to trigger physiological processes in plants. Nowadays, different priming techniques such as hydropriming, osmopriming, matrix priming, chemical priming, nutripriming, biopriming, and nanoparticle priming or coating seeds with nanoparticles (NPs) are being developed to provide better seed quality, strengthen seeds, accelerate the germination process, and minimize environmental stress [69]. Seed priming plays a role in the formation of stress response through the accumulation of osmolytes (proline, glycine-betaine, and polyamines) by affecting various biochemical processes (cell repair mechanism, antioxidant defense mechanism, etc.) [65].
3.1.1 Hydropriming
Hydropriming is the process of immersing seeds in water to make them stress-tolerant and then drying them to stop germination. This technique is cost-effective compared to other preparation techniques and does not cause toxicity and pollution problems for the seeds and the environment (Table 3). The duration of hydropriming is determined by the type of treated seed [66]. It was reported that seedling dry weight and germination percentage increased in hydroprimed basil (
| Seed priming method | Priming agent | Plant | Priming effect | Priming agent concentration/duration | Ref. |
|---|---|---|---|---|---|
| Hydropriming | Water | Drought tolerance | 36 h | [70] | |
| Osmopriming | PEG | Osmopriming improved stress tolerance of germinating seeds/increasing the germination potential of primed seeds | PEG 8000 (1, 2, 4 and 8 days/−0.6 MPa) | [71] | |
| KNO3 | Increased salt tolerance of sunflower seeds by promoting K+ and Ca2+ accumulation and inducing osmoregulation by the accumulation of proline | 24 h KNO3 (−1.0 MPa) | [72] | ||
| CaCl2 | Improved leaf area index, leaf area duration, and crop growth rate | −1.25 MPa | [73] | ||
| Melatonin | Improved seed germination under the drought stress, higher antioxidant activities | 500 mM 6 h | [74] | ||
| Chemical priming | Paclobutrazol | Drought tolerance | 50 mg/L−1 | [75] | |
| Chitosan | Low temperature tolerance | 0.50% (w/v) | [76] | ||
| Putrecine (Put) | Chilling tolerance | 0.01 mM and 0.1 mM put for 24 h and 48 h | [77] | ||
| Polyamines (putrecine, spermidine, spermine) | Drought tolerance | 10 μM | [78] | ||
| Spermidine (Spd) | Salt tolerance | 1 mM-soaked seeds for 14 days | [79] | ||
| NaHS and CaCl2 | Adaptation to Ni stress by increasing the AsA-GSH cycle, redox homeostasis | NaHS (100 μM) 24 h CaCI2 (15 mM) 24 h | [80] | ||
| Choline | Salt tolerance | 5 mM (24 h) | [81] | ||
| KH2PO4 | Increased germination percentage under salt stress | 0.5% (w/v) | [82] | ||
| Hormonal priming | Gibberellic acid | Drought tolerance | 300 mg/L 8 h | [83] | |
| Gibberellic acid, Salicylic acid | Salicylic acid priming greatly enhanced the drought stress tolerance | 10−4 M | [84] | ||
| Salicylic acid | Shortened the germination period and improved the germination rate | 50, 75, 100 ppm/12 h | [85] | ||
| Biopriming | Inoculation with | Salt tolerance | Cell density of 106 cells/ml | [86] | |
| Improving germination percentage and reducing reduction percentage of germination during salinity stress | 10 g/kg of seeds | [87] | |||
| Decrease in proline, MDA, and hydrogen peroxide | — | [88] | |||
| Nutripriming | Zinc (ZnSO4) | Higher antioxidant potential (SOD activity), better germination and development of seedlings | 9, 20 and 50 mg Zn kg1 | [89] | |
| Higher dissipation of excess energy, higher leaf succulence values | 0.4% Zn 8 h | [90] | |||
| Mg(NO3)2 and ZnSO4 | Higher yield and attributes parameters (spike length, spike weight, seed count) | — | [91] |
Table 3.
Seed priming-induced stress tolerance in plants.
3.1.2 Osmopriming
Osmopriming is a priming method that uses osmotic solutions and activates metabolic processes before germination by providing lower water potential to the seeds. This practice takes advantage of the slow absorption of water through osmopriming agents. Osmopriming agents include sodium chloride, potassium chloride, potassium nitrate, calcium chloride, magnesium sulfate, mannitol, sorbitol, PEG (polyethylene glycol), and glycerol (Table 3). PEG is commonly used as osmopriming agent due to its large molecular size and nontoxic structure [92, 93]. Osmopriming is a more rapidly applicable technique compared to hydropriming. It provides farmers with a very appealing alternative to develop crop establishment and yield. Moreover, it has significantly lower cost than conventional water saving strategies [92, 94].
3.1.3 Chemical priming
Numerous natural or synthetic chemicals such as paclobutrazol, butenolide, choline, paclobutrazol, and chitosan are preferred as chemical preparation components to ensure that physiological and biological processes are not disrupted in plants under stress conditions (Table 3). Treatment with butenolide increases the germination and development rate in
3.1.4 Biological priming
Microorganism-mediated biopriming (Plant Growth-Promoting Rhizobacteria (PGPRs), biocontrol agents, and fungicides) improves germination, seedling vigor, and biotic/abiotic stress tolerance and synchronizes crop stand. Microorganisms that are beneficial to plants such as
3.1.5 Hormonal priming
Hormonal priming involves the application of solutions containing plant growth regulators to the seeds. Various regulators such as abscisic acid (ABA), SA (salicylic acid), gibberellic acid (GA3), ascorbate, kinetin, auxin, cytokinin, gibberellin, ethylene, and polyamines are used for priming (Table 3). Hormonal priming of
3.1.6 Nutripriming
Nutripriming is a technique involving the application of solutions containing magnesium, zinc, and boron to the seeds. The mineral-nutrient status of plants plays a critical role in enhancing plant resistance to environmental stressors. Nutripriming with Zn in
3.2 Plant priming
In addition to numerous applications of priming to the seeds, plants can also get primed at the seedling stage. Soil irrigation and foliar spraying are the most widely used methods during priming application at seedling stage [64, 65]. Putrescine and spermine were applied to the wheat plants as chemical priming agents by spraying at the concentration of 0.1 mM. The mixture of putrescine and spermine not only improved
3.3 Priming and epigenetic
The ability of plants to recall previous stress after priming with biotic and abiotic stressors has been linked to epigenetic mechanisms. The accumulation of signaling molecules, DNA methylation, and histone modifications (HM) can be listed. For instance, histone deacetylases (HDACs) are involved in cellular processes such as chromatin structure and gene expression in the formation of stress responses to abiotic stresses by deacetylating histones. On the other hand, 5-methylcytosine (5mC), as another major epigenetic signal, is controlled by various DNA methyltransferases and demethylases. RNA-directed DNA methylation (RdDM) is an epigenetic process that mediates DNA methylation at specific DNA sequences of noncoding RNA. Together with DNA demethylation, RdDM plays a role in response to abiotic and biotic stresses by regulating the activity of transposable elements (TEs) and gene expression in both plant development and stress responses [104]. Recent studies have shown that epigenetic features such as histone acetylation and methylation can be altered by priming to alter chromatin state. This would alter transcriptional responses to a re-emerging stressor (Table 2). It has been suggested that stress exposure may modulate transcriptional responses [62]. High temperature stress has been associated with epigenetic marks such as methylation [5-methylcytosine (5mC), N6-methyladenine (6 mA)], siRNAs-controlled RdDMs, and histone modifications. DNA methylation mediated by miRNAs is involved in salinity and drought stresses. Small RNAs, especially miRNAs, also contribute to the intergenerational inheritance of heat stress in
4. Concluding remarks and prospects
In this chapter, we focused on seed or plant priming applications and their future in agriculture to increase tolerance to abiotic and biotic stress conditions in important crop plants and to describe the mechanisms of triggering, protecting, and removing the stress memory in plants. We may suggest that combining priming and stress memory studies will be much more advantageous in elucidating the mechanisms involved in improving the stress response of plants. Seed priming methods can be used to increase plant performance under stressful conditions. We also should underline the fact that further studies and insight are required in many areas such as epigenetics of plant growth, development, and stress response in order to use these methods with maximum efficiency in the field.
The discovery of the cognitive abilities of plants to form and retain memory has offered scientists and plant breeders exciting new opportunities. Primary stress exposure or stress priming, as a nongenetic approach, has been considered as one of the novel approaches to improve crop tolerance and resistance, regulating the genetic and epigenetic processes that govern the stress memory of plants. Plants can sense the conditions in their environment and integrate over time and store environmental cues. Plants become more responsive to the stresses that are repeated over and over in subsequent generations in conjunction with changes coordinated with stress memory at the cellular levels. Acquired tolerance refers to memory that occurs due to stress.
Priming has some potential to produce more tolerant and productive crops in the future. However, its relation to molecular mechanisms and stress memory has not yet been fully understood. Like the genome, transcriptome, and proteome of plants, their epigenome also contributes to stress responses. As a result, primed plants respond more rapidly and effectively to biotic or abiotic stress conditions. Stress priming, also known as training or conditioning, can increase short- or long-term stress memory of plants. This allows plants to get more resistant to more stresses in current and even subsequent generations. It has been shown in many studies that mitotic stress memory, which affects the somatic memory of a generation, and meiotic stress memory, which affects the memory of future generations, can play an important role in the development of plant responses to stresses. Somatic memory is typically transient and is activated when exposed to acute stress but can be reactivated typically within a limited time frame, that is, a few hours or days. Various priming strategies have been applied, such as accumulation of various cellular compounds, phosphorylation of mitogen-activated protein kinases (MAPKs), and modification of regulatory proteins through epigenetic mechanisms [53]. In plants, somatic memory is achieved through a variety of mechanisms such as chromatin remodeling, alternative transcript splicing, metabolite accumulation, and autophagy [105].
Besides advantages such as ease of application and cost-effectiveness, there are also disadvantages such as the risks of causing an increase in the amount of chemicals in agricultural areas and the difficulties in scale-up applications in large fields. Moreover, the necessity of dose optimization and application time depending on the variety is also a major obstacles. Priming synchronizes seed germination and facilitates seedling formation and plant growth under stressful conditions; hence, it supports yield increase. However, there are important gaps in the development of tolerance and resistance by stress priming and stress memory in plants. There are many aspects that need to be clarified in the future. Intergenerational memory, which is passed directly from the first generation to the next, can be reset in the second generation. As stress memory is passed on to offspring, it can be reset or cause negative impact in plants due to phenotypic or physiological stress plasticity. For this reason, in-field validation is essential, and more research is required to fully understand the whole stress memory regulation. Understanding the molecular basis of stress priming will contribute to identifying potential stress response options including cellular targets and signaling networks.
Seed priming is a pre-germination process and creates mild stress in the early stages of germination through various chemical, physical, and biological agents. To maximize the cost/benefit ratio for farmers, it is crucial to identify and cultivate the local varieties that best respond to seed priming. Contrary to the conditions in the laboratory, plants encounter many stresses in various combinations at the same time in their natural environment. Therefore, further studies are required to understand how epigenetic changes and responsible pathways are adjusted against different abiotic or biotic stresses, priming doses, and durations. Determination and optimization of the plant development stages to initiate priming can also enhance effectiveness and efficiency. Research on the regulation of epigenetic stress responses in crops needs to be accelerated. Data on DNA methylation should be evaluated with other epigenetic changes in establishing stress tolerance. Thus, the roles of epigenetic changes induced by stress in regulation of the expression of crucial genes involves signal perception or transduction. It should be considered that the development of plants under optimal conditions is somewhat compromised by seed priming to alter the somatic or transgenerational memory of plants. Crop productivity may be adversely affected as the quality of seeds with the best germination rate is reduced due to applied prestress. Their seedling growth may also potentially get interrupted. From this point of view, the balance between plant growth and stress tolerance should be carefully evaluated. With a variety of omics approaches (proteomics, metabolomics, transcriptomics, and epigenomics) and genome-wide association studies (GWASs) using high-throughput methods, it is possible to learn the molecular basis of stress memory of plants and generate data to correlate molecular function with agronomic performance.
When abiotic and biotic stresses occur, it is vital to recall molecular experiences and utilize recorded information to adapt to new conditions. Priming and stress memory are novel focal points for crop management and sustainable agriculture. While plants with rearranged stress memory offer a new possibilities for creating highly productive agricultural practices with better stress responses, it is an undeniable fact that much research is required to develop this strategy and apply it effectively in field.
References
- 1.
Ogruc Ildiz G, Celik O, Atak C, et al. Raman spectroscopic and chemometric investigation of lipid–protein ratio contents of soybean mutants. Applied Spectroscopy. 2020; 74 :34-41. DOI: 10.1177/00037028198599 - 2.
Çelik Ö, Ayan A, Meriç S, et al. Comparison of tolerance related proteomic profiles of two drought tolerant tomato mutants improved by gamma radiation. Journal of Biotechnology. 2021; 330 :35-44. DOI: 10.1016/j.jbiotec.2021.02.012 - 3.
Ayan A, Meriç S, Gümüş T, et al. In: Ohyama T, Takahashi Y, Ohtake N, Sato T, Tanabata S, editors. Current Strategies and Future of Mutation Breeding in Soybean Improvement, Soybean—Recent Advances in Research and Applications. London, UK: IntechOpen; 2022. DOI: 10.5772/intechopen.104796 - 4.
Meriç S, Ayan A, Atak Ç, et al. Profile-based proteomic investigation of unintended effects on transgenic and gamma radiation induced mutant soybean plants. Genetic Resources and Crop Evolution. 2023; 70 (2077-2095):1-19. DOI: 10.1007/s10722-023-01560-5 - 5.
Meriç S, Ayan A, Atak Ç. In: Fahad S, Saud S, Chen Y, Wu C, Wang D, editors. Molecular abiotic stress tolerans strategies: From genetic engineering to genome editing era, Abiotic Stress Plants. London, UK: IntechOpen; 2020. DOI: 10.5772/intechopen.94505 - 6.
Meriç S, Gümüş T, Ayan A. Plant-based vaccines: The future of preventive healthcare? In: Ghimire K, editor. Botany—Recent Advances and Applications. London, UK: IntechOpen; 2021. DOI: 10.5772/intechopen.97861 - 7.
Ayan A, Meriç S, Gümüş T, et al. Next generation of transgenic plants: From farming to pharming. In: Sithole Niang I, editor. Genetically Modified Plants and Beyond. London, UK, London, UK: IntechOpen; 2021. DOI: 10.5772/intechopen.102004 - 8.
Ayan A, Meriç S, Gümüş T, et al. Transgenic plants in heat stress adaptation: Present achievements and prospects. In: Oliveira M, Fernandes-Silva A, editors. Abiotic Stress in Plants—Adaptations to Climate Change. London, UK: IntechOpen; 2023. DOI: 10.5772/intechopen.111791 - 9.
Meriç S, Çakır Ö, Turgut-Kara N, et al. Detection of genetically modified maize and soybean in feed samples. Genetics and Molecular Research: GMR. 2014; 13 :1160-1168. DOI: 10.4238/2014.February.25.2 - 10.
Çakır Ö, Meriç S, Arı Ş. GMO analysis methods for food: From today to tomorrow. Food Safety. In: Si UG, Giuseppe C, editors. Food Safety: Innovative Analytical Tools for Safety Assessment. New York: WILEY-Scrivener Publishing; 2017. pp. 123-179. DOI: 10.1002/9781119160588 - 11.
Aranega-Bo P, de la O Leyva M, Finiti I, et al. Priming of plant resistance by natural compounds. Frontiers in Plant Science. 2014; 5 :488. DOI: 10.3389/fpls.2014.00488 - 12.
Harris CJ, Amtmann A, Ton J. Epigenetic processes in plant stress priming: Open questions and new approaches. Current Opinion in Plant Biology. 2023; 75 :102432. DOI: 10.1016/j.pbi.2023.102432 - 13.
Nosaka M, Itoh JI, Nagato Y, et al. Role of transposon-derived small RNAs in the interplay between genomes and parasitic DNA in Rice. PLoS Genetics. 2012; 8 :e1002953. DOI: 10.1371/journal.pgen.1002953 - 14.
Chang YN, Zhu C, Jiang J, et al. Epigenetic regulation in plant abiotic stress responses. Journal of Integrative Plant Biology. 2020; 62 :563-580. DOI: 10.1111/jipb.12901 - 15.
Lucibelli F, Valoroso MC, Aceto S. Plant DNA methylation: An epigenetic mark in development, environmental interactions, and evolution. International Journal of Molecular Sciences. 2022; 23 :8299. DOI: 10.3390/ijms23158299 - 16.
Li M, Da FY. Histone variants: The artists of eukaryotic chromatin. Science China Life Sciences. 2015; 58 :232-239. DOI: 10.1007/s11427-015-4817-4 - 17.
Struhl K, Segal E. Determinants of nucleosome positioning. Nature Structural & Molecular Biology. 2013; 20 :267-273. DOI: 10.1038/nsmb.2506 - 18.
Foroozani M, Holder DH, Deal RB. Histone variants in the specialization of plant chromatin. Annual Review of Plant Biology. 2022; 73 :149-172. DOI: 10.1146/annurev-arplant-070221-050044 - 19.
Zhao T, Zhan Z, Jiang D. Histone modifications and their regulatory roles in plant development and environmental memory. Journal of Genetics and Genomics. 2019; 46 :467-476. DOI: 10.1016/j.jgg.2019.09.005 - 20.
Zhang C, Du X, Tang K, et al. Arabidopsis AGDP1 links H3K9me2 to DNA methylation in heterochromatin. Nature Communications. 2018; 9 :1-14. DOI: 10.1038/s41467-018-06965-w - 21.
Brewis HT, Wang AY, Gaub A, et al. What makes a histone variant a variant: Changing H2A to become H2A.Z. PLoS Genetics. 2021; 17 :e1009950. DOI: 10.1371/journal.pgen.1009950 - 22.
Liu H, Able AJ, Able JA. Small RNAs and their targets are associated with the transgenerational effects of water-deficit stress in durum wheat. Scientific Reports. 2021; 11 :1-17. DOI: 10.1038/s41598-021-83074-7 - 23.
Kambona CM, Koua PA, Léon J, et al. Stress memory and its regulation in plants experiencing recurrent drought conditions. Theoretical and Applied Genetics. 2023; 136 :1-21. DOI: 10.1007/s00122-023-04313-1 - 24.
Bhadouriya SL, Mehrotra S, Basantani MK, et al. Role of chromatin architecture in plant stress responses: An update. Frontiers in Plant Science. 2021; 11 :603380. DOI: 10.3389/fpls.2020.603380 - 25.
Review M, Song ZT, Liu JX, et al. Chromatin remodeling factors regulate environmental stress responses in plants. Journal of Integrative Plant Biology. 2021; 63 :438-450. DOI: 10.1111/jipb.13064 - 26.
Kim JH. Multifaceted chromatin structure and transcription changes in plant stress response. International Journal of Molecular Sciences. 2021; 22 :2013. DOI: 10.3390/ijms22042013 - 27.
Liu N, Staswick PE, Avramova Z. Memory responses of jasmonic acid-associated Arabidopsis genes to a repeated dehydration stress. Plant, Cell & Environment. 2016; 39 :2515-2529. DOI: 10.1111/pce.12806 - 28.
Ding Y, Fromm M, Avramova Z. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nature Communications. 2012; 3 :1-9. DOI: 10.1038/ncomms1732 - 29.
Crisp PA, Ganguly D, Eichten SR, et al. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Science Advances. 2016; 2 :e1501340. DOI: 10.1126/sciadv.150134 - 30.
de Freitas A, Guedes F, Menezes-Silva PE, DaMatta FM, et al. Using transcriptomics to assess plant stress memory. Theoretical and experimental. Plant Physiology. 2019; 31 :47-58. DOI: 10.1007/s40626-018-0135-0 - 31.
Métraux JP, Signer H, Ryals J, et al. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990; 250 :1004-1006. DOI: 10.1126/science.250.4983.10 - 32.
Jacques C, Salon C, Barnard RL, et al. Drought stress memory at the plant cycle level: A review. Plants. 2021; 10 :1873. DOI: 10.3390/plants10091873 - 33.
Lämke J, Bäurle I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biology. 2017; 18 :1-11. DOI: 10.1186/s13059-017-1263-6 - 34.
Sharma M, Kumar P, Verma V, et al. Understanding plant stress memory response for abiotic stress resilience: Molecular insights and prospects. Plant Physiology and Biochemistry. 2022; 179 :10-24. DOI: 10.1016/j.plaphy.2022.03.004 - 35.
Zuo DD, Ahammed GJ, Guo DL. Plant transcriptional memory and associated mechanism of abiotic stress tolerance. Plant Physiology and Biochemistry. 2023; 201 :107917. DOI: 10.1016/j.plaphy.2023.107917 - 36.
Oberkofler V, Pratx L, Bäurle I. Epigenetic regulation of abiotic stress memory: Maintaining the good things while they last. Current Opinion in Plant Biology. 2021; 61 :102007. DOI: 10.1016/j.pbi.2021.102007 - 37.
Jaskiewicz M, Conrath U, Peterhälnsel C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Reports. 2011; 12 :50-55. DOI: 10.1038/embor.2010.186 - 38.
Brzezinka K, Altmann S, Czesnick H, et al. Arabidopsis FORGETTER1 mediates stress-induced chromatin memory through nucleosome remodeling. eLife. 2016; 5 :e17061. DOI: 10.7554/eLife.17061 - 39.
Wibowo A, Becker C, Marconi G, et al. Hyperosmotic stress memory in arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by dna glycosylase activity. eLife. 2016; 5 :e13546. DOI: 10.7554/eLife.13546 - 40.
Kuźnicki D, Meller B, Arasimowicz-Jelonek M, et al. BABA-induced DNA methylome adjustment to intergenerational defense priming in potato to Phytophthora infestans. Frontiers in Plant Science. 2019; 10 :445885. DOI: 10.3389/fpls.2019.00650 - 41.
Whittle CA, Otto SP, Johnston MO, et al. Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana . Botany. 2009;87 :650-657. DOI: 10.1139/B09-030 - 42.
Nosalewicz A, Siecińska J, Śmiech M, et al. Transgenerational effects of temporal drought stress on spring barley morphology and functioning. Environmental and Experimental Botany. 2016; 131 :120-127. DOI: 10.1016/j.envexpbot.2016.07.006 - 43.
Hatzig SV, Nuppenau JN, Snowdon RJ, et al. Drought stress has transgenerational effects on seeds and seedlings in winter oilseed rape ( Brassica napus L.). BMC Plant Biology. 2018;18 :1-13. DOI: 10.1186/s12870-018-1531-y - 44.
Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science. 2010; 330 :612-616. DOI: 10.1126/science.1191078 - 45.
Ali M, Sugimoto K, Ramadan A, et al. Memory of plant communications for priming anti-herbivore responses. Scientific Reports. 2013; 3 :1872. DOI: 10.1038/srep01872 - 46.
Berry S, Dean C. Environmental perception and epigenetic memory: Mechanistic insight through FLC. The Plant Journal. 2015; 83 :133-148. DOI: 10.1111/tpj.12869 - 47.
Hilker M, Schmülling T. Stress priming, memory, and signalling in plants. Plant, Cell & Environment. 2019; 42 :753-761. DOI: 10.1111/pce.13526 - 48.
Avramova Z. Transcriptional ‘memory’ of a stress: Transient chromatin and memory (epigenetic) marks at stress-response genes. The Plant Journal. 2015; 83 :149-159. DOI: 10.1111/tpj.12832 - 49.
Hauser MT, Aufsatz W, Jonak C, et al. Transgenerational epigenetic inheritance in plants. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms. 2011; 1809 :459, 10.1016/j.bbagrm.2011.03.007 - 50.
Sedaghatmehr M, Thirumalaikumar VP, Kamranfar I, et al. A regulatory role of autophagy for resetting the memory of heat stress in plants. Plant, Cell & Environment. 2019; 42 :1054-1064. DOI: 10.1111/pce.13426 - 51.
Chen H, Dong J, Wang T. Autophagy in plant abiotic stress management. International Journal of Molecular Sciences. 2021; 22 :22. DOI: 10.3390/ijms22084075 - 52.
Calarco JP, Borges F, Donoghue MTA, et al. Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell. 2012; 151 :194-205. DOI: 10.1016/j.cell.2012.09.001 - 53.
Turgut-Kara N, Arikan B, Celik H. Epigenetic memory and priming in plants. Genetica. 2020; 148 :47-54. DOI: 10.1007/s10709-020-00093-4 - 54.
Singh RR, Pajar JA, Audenaert K, et al. Induced resistance by ascorbate oxidation involves potentiating of the phenylpropanoid pathway and improved rice tolerance to parasitic nematodes. Frontiers in Plant Science. 2021; 12 :713870. DOI: 10.3389/fpls.2021.713870 - 55.
Yang Z, Zhi P, Chang C. Priming seeds for the future: Plant immune memory and application in crop protection. Frontiers in Plant Science. 2022; 13 :961840. DOI: 10.3389/fpls.2022.961840 - 56.
Liu H, Able AJ, Able JA. Priming crops for the future: Rewiring stress memory. Trends in Plant Science. 2022; 27 :699-716. DOI: 10.1016/j.tplants.2021.11.015 - 57.
Luna E, Bruce TJA, Roberts MR, et al. Next-generation systemic acquired resistance. Plant Physiology. 2012; 158 :844-853. DOI: 10.1104/pp.111.187468 - 58.
Martínez-Aguilar K, Hernández-Chávez JL, Alvarez-Venegas R. Priming of seeds with INA and its transgenerational effect in common bean ( Phaseolus vulgaris L.) plants. Plant Science. 2021;305 :110834. DOI: 10.1016/j.plantsci.2021.110834 - 59.
Catoni M, Alvarez-Venegas R, Worrall D, et al. Long-lasting defence priming by β-aminobutyric acid in tomato is marked by genome-wide changes in DNA methylation. Frontiers Plant Science. 2022; 13 :836326. DOI: 10.3389/fpls.2022.836326 - 60.
Meller B, Kuźnicki D, Arasimowicz-Jelonek M, et al. Baba-primed histone modifications in potato for intergenerational resistance to phytophthora infestans. Front Plant Science. 2018; 9 :1228. DOI: 10.3389/fpls.2018.01228 - 61.
Sani E, Herzyk P, Perrella G, et al. Hyperosmotic priming of Arabidopsis seedlings establishes a long-term somatic memory accompanied by specific changes of the epigenome. Genome Biology. 2013; 14 :1-24. DOI: 10.1186/gb-2013-14-6-r59 - 62.
Yung WS, Wang Q , Huang M, et al. Priming-induced alterations in histone modifications modulate transcriptional responses in soybean under salt stress. Plant Journal. 2022; 109 :1575-1590. DOI: 10.1111/tpj.15652 - 63.
Cong W, Miao Y, Xu L, et al. Transgenerational memory of gene expression changes induced by heavy metal stress in rice ( Oryza sativa L.). BMC Plant Biology. 2019;19 :1-14. DOI: 10.1186/s12870-019-1887-7 - 64.
Jisha KC, Vijayakumari K, Puthur JT. Seed priming for abiotic stress tolerance: An overview. Acta Physiologiae Plantarum. 2012; 35 :1381-1396. DOI: 10.1007/s11738-012-1186-5 - 65.
Marthandan V, Geetha R, Kumutha K, et al. Seed priming: A feasible strategy to enhance drought tolerance in crop plants. International Journal of Molecular Sciences. 2020; 21 :8258. DOI: 10.3390/ijms21218258 - 66.
Devika OS, Singh S, Sarkar D, et al. Seed priming: A potential supplement in integrated resource management under fragile intensive ecosystems. Frontiers in Sustainable Food Systems. 2021; 5 :654001. DOI: 10.3389/fsufs.2021.654001 - 67.
Pawar VA, Laware SL. Seed priming a critical review. International Journal of Scientific Research in Biological Sciences. 2018; 5 :94-101 - 68.
Villagómez-Aranda AL, Feregrino-Pérez AA, García-Ortega LF, et al. Activating stress memory: Eustressors as potential tools for plant breeding. Plant Cell Reports. 2022; 41 :1481-1498. DOI: 10.1007/s00299-022-02858-x - 69.
Lal SK, Kumar S, Sheri V, et al. Seed priming: An emerging technology to impart abiotic stress tolerance in crop plants. In: Rakshit A, Singh H, editors. Advances in Seed Priming. Springer; 2018. pp. 41-50. DOI: 10.1007/978-981-13-0032-5_3 - 70.
Janmohammadi M, Dezfuli P, Physiol FS-GAP, et al. Seed invigoration techniques to improve germination and early growth of inbred line of maize under salinity and drought stress. General and Applied Plant Physiology. 2008; 34 :215-226 - 71.
Chen K, Arora R. Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in spinach ( Spinacia oleracea ). Plant Science. 2011;180 :212-220. DOI: 10.1016/j.plantsci.2010.08.007 - 72.
Bajehbaj AA. The effects of NaCl priming on salt tolerance in sunflower germination and seedling grown under salinity conditions. African Journal of Biotechnology. 2010; 9 :1764-1770. DOI: 10.5897/AJB10.1019 - 73.
Hussain M, Farooq M, Sattar A, et al. Mitigating the adverse effects of drought stress through seed priming and seed quality on wheat ( Triticum aestivum L.) productivity. Pakistan Journal of Agricultural Sciences. 2023;55 :313-319. DOI: 10.21162/PAKJAS/185833 - 74.
Khan MN, Zhang J, Luo T, et al. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Industrial Crops and Products. 2019; 140 :111597. DOI: 10.1016/j.indcrop.2019.111597 - 75.
Souza-Machado V, Pitblado R, Ali A, et al. Paclobutrazol in tomato ( Lycopersicon esculentum ) for improved tolerance to early transplanting and earlier harvest maturity. Acta Horticulture. 1999;487 :139-143. DOI: 10.17660/ActaHortic.1999.487.17 - 76.
Guan YJ, Hu J, Wang XJ, et al. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. Journal of Zhejiang University Science B. 2009; 10 :427-433. DOI: 10.1631/jzus.B0820373 - 77.
Xu S, Hu J, Li Y, et al. Chilling tolerance in Nicotiana tabacum induced by seed priming with putrescine. Plant Growth Regulation. 2011; 63 :279-290. DOI: 10.1007/s10725-010-9528-z - 78.
Farooq M, Wahid A, Lee DJ. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiologiae Plantarum. 2009; 31 :937-945. DOI: 10.1007/s11738-009-0307-2 - 79.
Chunthaburee S, Sanitchon J, Pattanagul W, et al. Alleviation of salt stress in seedlings of black glutinous rice by seed priming with spermidine and gibberellic acid. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2014; 42 :405-413. DOI: 10.15835/nbha4229688 - 80.
Valivand M, Amooaghaie R, Ahadi A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiology and Biochemistry. 2019; 143 :286-298. DOI: 10.1016/j.plaphy.2019.09.016 - 81.
Salama K, Mansour M, Sci NH-AJBA, et al. Choline priming improves salt tolerance in wheat ( Triticum aestivum L.). Australian Journal of Basic and Applied Sciences. 2011;5 :126-132. DOI: 10.13140/2.1.4228.9606 - 82.
Yagmur M, Kaydan D. Alleviation of osmotic stress of water and salt in germination and seedling growth of triticale with seed priming treatments. African Journal of Biotechnology. 2008; 7 :2156-2162 - 83.
Li Z, Lu GY, Zhang XK, et al. Improving drought tolerance of germinating seeds by exogenous application of gibberellic acid (GA3) in rapeseed ( Brassica napus L.). Seed Science and Technology. 2010;38 :432-440. DOI: 10.15258/sst.2010.38.2.16 - 84.
Ulfat A, Majid S, Bot AH-PJ, et al. Hormonal seed priming improves wheat ( Triticum aestivum L.) field performance under drought and non-stress conditions. Pakistan Journal of Botany. 2017;49 :1239-1253 - 85.
Jatana BS, Ram H, Gupta N. Application of seed and foliar priming strategies to improve the growth and productivity of late sown wheat ( Triticum aestivum L.). Cereal Research Communications. 2020;48 :383-390. DOI: 10.1007/s42976-020-00036-x - 86.
Bano A, Fatima M. Salt tolerance in Zea mays (L). Following inoculation with Rhizobium and Pseudomonas. Biology and Fertility of Soils. 2009;45 :405-413. DOI: 10.1007/s00374-008-0344-9 - 87.
Rawat L, Singh Y, Shukla N, et al. Alleviation of the adverse effects of salinity stress in wheat ( Triticum aestivum L.) by seed biopriming with salinity tolerant isolates ofTrichoderma harzianum . Plant and Soil. 2011;347 :387-400. DOI: 10.1007/s11104-011-0858-z - 88.
Shukla N, Awasthi RP, Rawat L, et al. Seed biopriming with drought tolerant isolates of Trichoderma harzianum promote growth and drought tolerance inTriticum aestivum . Annals of Applied Biology. 2015;166 :171-182. DOI: 10.1111/aab.12160 - 89.
Candan N, Cakmak I, Ozturk L. Zinc-biofortified seeds improved seedling growth under zinc deficiency and drought stress in durum wheat. Journal of Plant Nutrition and Soil Science. 2018; 181 :388-395. DOI: 10.1002/jpln.201800014 - 90.
Pavia I, Roque J, Rocha L, et al. Zinc priming and foliar application enhances photoprotection mechanisms in drought-stressed wheat plants during anthesis. Plant Physiology and Biochemistry. 2019; 140 :27-42. DOI: 10.1016/j.plaphy.2019.04.028 - 91.
Kumar Singhal R, Pradesh U, Vivek Kumar I, et al. Improving the yield and yield attributes in wheat crop using seed priming under drought stress. Journal of Pharmacognosy and Phytochemistry. 2019; 8 :214-220 - 92.
Bhanuprakash K, Yogeesha HS. Seed priming for abiotic stress tolerance: An overview. In: Rao N, Shivashankara K, Laxman R, editors. Abiotic Stress Physiology of Horticultural Crops. Springer; 2016. pp. 103-117. DOI: 10.1007/978-81-322-2725-0_6 - 93.
Rhaman MS, Imran S, Rauf F, et al. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants. 2021; 10 :37. DOI: 10.3390/plants10010037 - 94.
Farooq M, Hussain M, Habib M. Influence of seed priming techniques on grain yield and economic returns of bread wheat planted at different spacings. Crop and Pasture Science. 2020; 71 :725-738. DOI: 10.1071/cp20065 - 95.
Savvides A, Ali S, Tester M, et al. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Science. 2016; 21 :329-340. DOI: 10.1016/j.tplants.2015.11.003 - 96.
Chakraborti S, Bera K, Sadhukhan S, et al. Bio-priming of seeds: Plant stress management and its underlying cellular, biochemical and molecular mechanisms. Plant Stress. 2022; 3 :100052. DOI: 10.1016/j.stress.2021.100052 - 97.
Mishra M, Kumar Madan Mohan U, Prakash V, et al. Efficiency of plant growth promoting rhizobacteria for the enhancement of Cicer arietinum L. growth and germination under salinity. Advances in Biological Research. 2010;4 :92-96 - 98.
Mangena P. Effect of hormonal seed priming on germination, growth, yield and biomass allocation in soybean grown under induced drought stress. Indian Journal of Agricultural Research. 2020; 54 :592-598. DOI: 10.18805/IJARe.A-441 - 99.
Singh Shivay Y, Prasad R, Pal M. Genetic variability for zinc use efficiency in chickpea as influenced by zinc fertilization. International Journal of Bio-resource Stress Management. 2014; 5 :031-036. DOI: 10.5958/j.0976-4038.5.1.005 - 100.
Tavili A, Zare S, Enayati A. Hydropriming, ascorbic and salicylic acid influence on germination of Agropyron elongatum host. Seeds under salt stress. Research Journal of Seed Science. 2009;2 :16-22. DOI: 10.3923/rjss.2009.16.22 - 101.
Hassan N, Ebeed H, Aljaarany A. Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiology and Molecular Biology of Plants. 2020; 26 :233-245. DOI: 10.1007/s12298-019-00744-7 - 102.
Bagheri M, Gholami M, Baninasab B. Hydrogen peroxide-induced salt tolerance in relation to antioxidant systems in pistachio seedlings. Scientia Horticulturae. 2019; 243 :207-213. DOI: 10.1016/j.scienta.2018.08.026 - 103.
Wei LX, Lv BS, Wang MM, et al. Priming effect of abscisic acid on alkaline stress tolerance in rice ( Oryza sativa L.) seedlings. Plant Physiology and Biochemistry. 2015;90 :50-57. DOI: 10.1016/j.plaphy.2015.03.002 - 104.
Gallusci P, Agius DR, Moschou PN, et al. Deep inside the epigenetic memories of stressed plants. Trends Plant Science. 2023; 28 :142-153. DOI: 10.1016/j.tplants.2022.09.004 - 105.
Srivastava AK, Suresh Kumar J, Suprasanna P. Seed ‘primeomics’: Plants memorize their germination under stress. Biological Reviews. 2021; 96 :1723-1743. DOI: 10.1111/brv.12722