Chemical composition of acid-leached SCBA from XRF [9].
Abstract
Silica molecules present in commercial objects can pose a hazard to human health, which is why the environmentally friendly synthesis of silica has been intensively researched in the recent decades. This chapter describes the synthesis of silica from sugarcane bagasse waste and its physical and chemical properties for potential use in eco-friendly applications. Sugarcane bagasse was burned to produce ash, which was then calcined in a 700°C kiln before being treated with citric acid to remove silica from the ash. X-ray fluorescence (XRF) analysis showed that after the acid treatment, 78–79% of the silica was produced and strong peaks were observed in the X-ray diffraction spectra (XRD) at 2Ɵ = 28 (degree) and an average diameter of 28 nm for 1-HDTA and 30 nm for TPAH, determined by the Scherrer equation. Fourier transform infrared spectroscopy (FTIR) spectra also confirms the presence of synthesized silica. In addition, the shape of the particles was analyzed by TEM and SEM images and it is found that synthesized silica had a spongy shape with irregular sizes ranging from 25 to 50 nm. Overall, the studies show that organic bases are capable of synthesizing silica with application-specific properties from agricultural waste using green chemistry.
Keywords
- green synthesis
- biomass
- nanomaterials
- agricultural waste
- environmentally friendly
1. Introduction
The synthesis of various nanomaterials for the development of nanoelectronics has sparked a great deal of interest in nanotechnology as a dependable, sustainable, and green process known as “green synthesis.” Green synthesis is essentially seen as a significant way to mitigate the issues with traditional methods for creating nanoparticles for use in functional nanomaterials and nanoelectrodes. The four most important variables in the synthesis of nanoparticles according to the green protocol are known to be the selection of an environmentally friendly solvent, a source of nanomaterials, a reducing agent, and a safe material for stabilization [1, 2].
Green chemistry strives to reduce the negative impacts of nanomaterial manufacturing and application by delivering risk-free nanotechnology. Green synthesis is a breakthrough in a variety of new technologies, including energy-saving procedures, large-scale manufacturing of nanoparticles, biological systems, and, finally, an eco-friendly method that does not use poisonous chemicals. A plethora of items have hit the market in recent years, with a select few being integrated into cosmetics, care products, and clothes merely because they are safe to use [2].
The nanomaterials synthesis from agricultural waste is thought to be environmentally friendly. The disposal of sugarcane bagasse attracts a large number of insects that are dangerous to human health and spread a wide range of diseases. This feedstock is used for the production of sugar and ethanol and the residue from the process is disposed as bagasse. The agricultural waste is disposed of in landfills, the majority of agricultural operations produce waste that is produced in large quantities in many countries and poses serious environmental issues [3].
Due to anaerobic decay, organic wastes produce carbon dioxide (CO2), nitrous oxide (N2O), and primarily methane (CH4) in landfills, all of which actively contribute to greenhouse gas emissions. Furthermore, during agricultural production, greenhouse gases primarily CH4 and N2O are emitted, with only a trace amount of CO2 emitted. Reusing agricultural solid waste is regarded as an important waste reduction strategy that meets the requirements of a comprehensive sustainable waste management system; reuse of these wastes can actively contribute to the development of new green technologies, bio-energy generation, and bio-conversion to nanomaterials [3, 4].
Numerous types of nanomaterials have been extracted from agricultural wastes using biogenic synthesis; these synthesis methods are an appealing alternative to conventional synthesis methods because they are green and environmentally friendly. Nanotechnology has emerged as one of the most important branches of science, with numerous industrial applications ranging from the conversion of biomass into valuable materials in niche applications to the production of new materials. The green synthesis of silver nanoparticles (AgNPs) is an excellent example of this; this method employs biomolecules found in plants and agricultural wastes that act as reducing and stabilizing agents. Other noteworthy biogenic processes include the environmentally friendly production of biodegradable polyurethane using castor oil and the production of copper-doped zinc oxide (ZnO) nanoparticles from
Plants are viewed as low-cost, non-conservative natural chemical factories. Silver (Ag) can be biosynthesized using waste materials such as safflower (
Green synthesis also has the advantages of a feasible methodology, non-hazardous and viable procedures, and a wide range of applications in nanotechnology, biomedicine, and nano-optoelectronics. Because it is chemically inert and has a high melting point, silica is widely used in industry. This makes it highly functional with control over specific properties. The green synthesis of silica appears to be an important field of study with a lot of future growth potential.
2. Materials and methods
The chemicals used in the preparation were purchased from Sigma-Aldrich and were not purified further. Sigma-Aldrich (St. Louis, MO, USA) provided (1-Hexadecyl) trimethyl-ammonium bromide (98%), citric acid (99.5%), and tetrapropylammonium hydroxide (1.0 M) in H2O. The Sugar Illovo South Africa Company (KwaZulu-Natal, South Africa) supplied the sugarcane. Sugarcane bagasse was soaked in double-deionized water for 24 hours in a typical preparation procedure to remove dust and soil particles. The soaked sugarcane was then oven-dried for 6 hours. The sugarcane bagasse was then burned in the open air to produce black sugarcane bagasse ash (SCBA) Figure 1. Deionized water from the Milli-Q water purification system (Millipore, Bedford, MA, USA) was used in the synthesis.
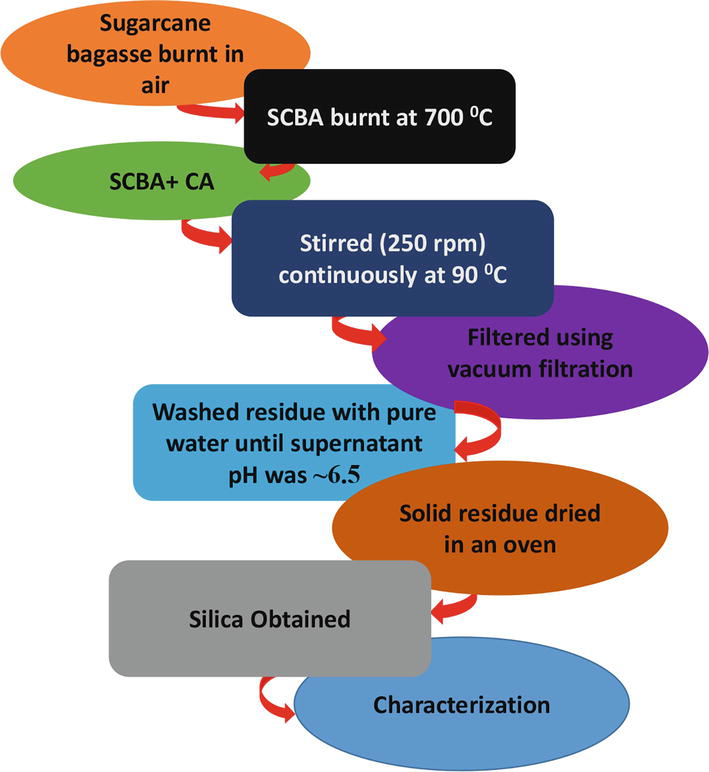
Figure 1.
Schematic illustration for the production of silica from sugarcane bagasse ash.
The bagasse ash was mixed with citric acid in a 250 ml and refluxed while stirring at 250 revolutions per minute (rpm) at 90°C for 2 hours, as shown in Figure 1. After that, the ash was washed twice with deionized water and decanted until the pH of the supernatant reached 6.5. The resulting ash was then dried in a 40°C oven overnight before being ground into a fine powder with a mortar and pestle. Finally, the acid leached bagasse is calcined in a furnace for four hours at 700°C.
In a typical hydrothermal reaction, two mL of tetrapropylammonium hydroxide were added to the orange-brown multicrystalline product shown in Eq. (1). The solution was then hydrothermally heated in a muffle furnace for 2 hours at 200°C, followed by 1 hour at 600°C in air to burn away the excess carbon, nitrogen, and hydrogen, as shown in Eq. (2). The resultant compound oxidizes C, H, and N to produce oxides and decomposes. As a result, 1-HDTA silica and TPAH-silica nanopowders were synthesized.
3. Results and discussion
The chemical composition of the SCBA was determined using X-ray fluorescence spectroscopy (XRF, Malvern Panalytical, Malvern, UK) for the major oxides listed in Table 1. According to the XRF analysis, the leaching process was efficient because the amount of silica was significantly reported to be 79.40 wt% and 78.79 wt% for the two organic acids, respectively. The leaching acid used in the pretreatment step was L-cysteine hydrochloride monohydrate. Table 1 also shows the loss on ignition. The significant improvement in silica morphology in ash powders is due to the selective removal of synthesis residues using L-cysteine hydrochloride monohydrate, essentially reducing metallic impurities in the sample. Following acid treatment, the sample was calcined in a muffle furnace, and silica was extracted using a hydro-thermal method with tetrapropylammonium hydroxide, minimizing other metal oxide impurities.
| Component | L-cys (wt%) | CA (wt%) |
|---|---|---|
| 79.40 | 78.79 | |
| 1.03 | 0.70 | |
| 8.45 | 8.25 | |
| 2.39 | 3.77 | |
| 0.04 | 0.04 | |
| 0.61 | 0.72 | |
| 1.06 | 1.26 | |
| 1.06 | 1.01 | |
| 3.86 | 3.66 | |
| 0.22 | 0.34 | |
| 0.03 | 0.01 | |
| 0.04 | 0.02 | |
| 0.02 | 0/01 | |
| 0.04 | −0.13 | |
| 1.18 | 1.50 |
Table 1.
3.1 Green silica synthesis
Green and sustainable approaches to obtaining advanced materials for industrial use can help avoid complex procedures while also reducing environmental toxins. Green synthesis methods have been investigated for the extraction of silica from various agricultural biomass sources. Because organic acids, alkalis, and solvents can decompose more complex-structured substrates, silica extraction from biomass is one of the most promising methods for green synthesis. One of the most promising methods for the green synthesis of silica and silicon nanoparticles is the chemical and thermal treatment of biomass by replacing toxic reactants and decreasing temperatures [9, 10].
3.2 Characterization
X-ray diffraction (XRD) on a Bruker AXSD8 Advancement equipment (Ithemba Labs, South Africa) with Cu-K1, λ = 154,050 A was used to cross-check the crystallinity identification. The Bragg angle array has a scanning step of 0.035°C and a temperature range of 2Ɵ = 10–90°C. IR was used to investigate the new surface functions by detecting the functional groups and bonding of elements present in the samples. The examination was performed at room temperature using a PerkinElmer FTIR spectrometer (spectrum 2) with a wavelength range of 400–4000 cm1. The surface morphology of SCBA was examined using scanning electron microscopy (TESCAN, VEGA). Before analysis, the samples were prepared on an aluminum stub and carbon sputtered on a carbon coater. Transmission Electron Microscopy (TEM) was used to study the structural morphology of the as-synthesized nanoparticles in order to assess particle size distribution and form.
Using Scherrer’s formula, the average diameter sizes of silica extracted with (1-Hexadecyl) trimethyl-ammonium bromide, (1-HDTA), and Tetrapropylammonium hydroxide (TPAH) were determined to be around 28 nm and 30 nm, respectively. Scherrer’s formula was used to estimate crystallite size, as stated in Eq. (3) below:
Dp is the average crystallite size (nm), and K is the Scherrer constant. K values range from 0.68 to 2.08, with K = 0.94 being preferred for spherical crystallites with cubic symmetry and -X-ray wavelength. CuKα the λ = 1.54178 is commonly used for micro XRD. Furthermore, the peak position is identified by the FWHM (full Width at Half Maximum) of the XRD peak calculated from one half of 2.
3.2.1 Powder X-ray diffraction analysis
Powder XRD was used to determine the structural characteristics of the powder. The SCBA was reacted with two distinct organic bases, 1-HDTA and TPAH, resulting in crystalline silica with average diameters of 28 nm for 1-HDTA and 30 nm for TPAH. This study effectively demonstrated the utilization of organic compounds for silica extraction from SCBA via a green synthesis technique [9]. The nature of crystallized phases is determined by measuring the angles of diffraction of X-rays by the solid’s crystalline plane. These diffraction angles are connected to the amorphous and crystal lattice properties. Figure 2a demonstrates that SCBA is primarily amorphous, with weak intensities of crystalline peaks present in the phase composition.
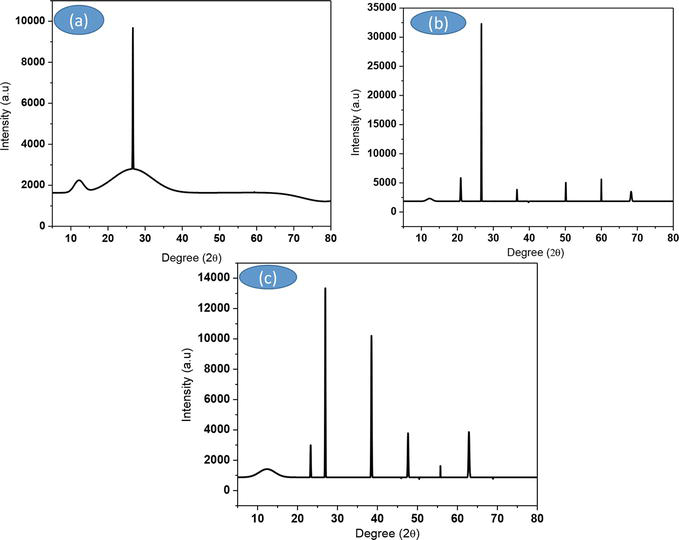
Figure 2.
The XRD patterns for (a) raw bagasse, (b) 1-HDTA silica, and (c) TPAH silica.
Diffraction peaks at 2 theta angles of 22, 28, 39, 48, 56, and 64 were observed in the XRD spectra of (b) 1-HDTA silica and (c) TPAH silica, corresponding to the crystalline phases of silica. The diffraction peak at 2 theta angle 28 reveals that the generated silica nanoparticles have a 101 cubic phase crystalline structure. Similarly, Katare, V.D., and Madurwar, M.V. (2017) [11] observed crystalline silica peaks at 2Ɵ = 26 [12]. This finding confirms that silica nanoparticles were extracted from SCBA in our samples [9, 11].
3.2.2 FTIR analysis
Figure 3b and c mention the characteristic peak number in the IR of the produced nano-silicas, display bands at 461.231 cm−1, 787.381 cm−1, and 1045.99 cm−1, which correspond to the Si∼O∼Si bending vibration, Si∼O∼Si stretch vibration, and Si∼O∼Si stretching vibration, respectively.
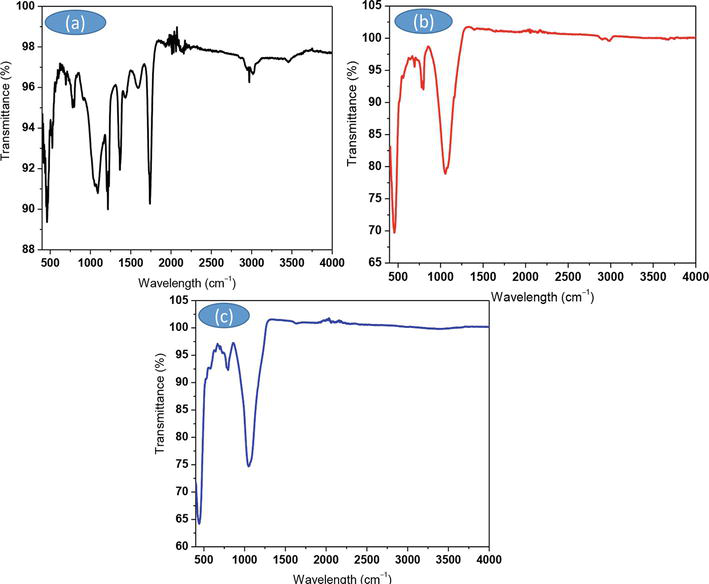
Figure 3.
FTIR spectra of (a) raw SCBA (b) 1-HDTA silica, and (c) TPAH silica.
The evolution and disappearance of functional groups, as well as the appearance of more prominent groups, demonstrate the incorporation of new functionalities in our samples. The carbonyl group band is visible in Figure 3a, but not in the silica spectra of either sample in (b) or (c), confirming the effective conversion of SCBA into silica groups, which are prevalent in the material. The band at 3014.44 cm−1 also corresponds to the C-H stretch in (a). SCBA has a weak absorption band for the hydroxyl group (OH) at about 3445.23 cm−1 [11].
The nanosilicas produced in (b) and (c) exhibit identical behavior for novel functionality. Despite the impressive results, the silica in Figure 3c displayed a generally intense and prominent band at roughly 1060.76 cm−1, which is typical of crystalline silica and confirms the results obtained from XRD diffraction patterns [11].
3.2.3 TEM and SEM analysis
TEM determines the particle size and shape of the materials. Figure 4a depicts the intensive particle aggregation in SCBA, as well as the produced silica in (b) and (c). Because the particle size is not consistent, it does not represent single particles but rather nanoscale agglomerates (50–100 nm). The silica particles are uniformly dispersed after the TPAH reaction. The particle sizes of the obtained silica and the acid leached using 1-HDTA are distributed randomly. The morphology of the nanosilica derived from the SCBA is identical to that obtained and reported elsewhere [13]. In contrast, agglomerated particles are usually in the nanorange. In terms of size control, this method of green synthesis of nano-silica particles is promising; nonetheless, non-uniform silica nanoparticles were also created.
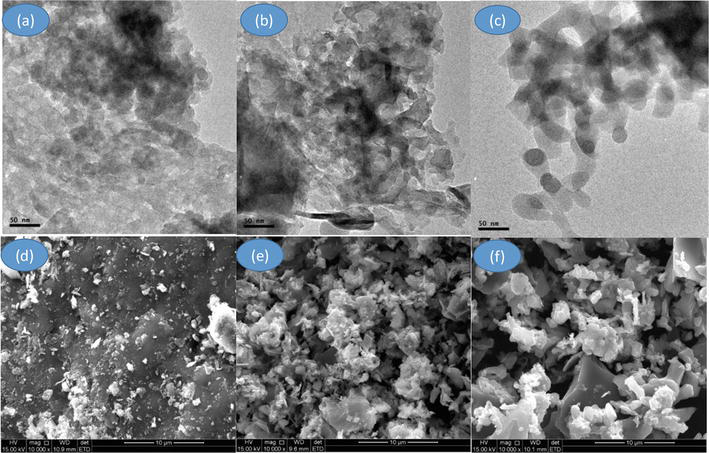
Figure 4.
TEM images of (a) raw SCBA, (b) 1-HDTA-silica, (c) TPAH silica and SEM images of (d) raw SCBA, (e) 1-HDTA and (f) TPAH silica.
In all samples linked with the release of organic matter during bagasse burning, the SEM images in Figure 4d–f indicate agglomerates of heterogeneous material with irregular forms and high roughness. Although the organic acid leaching process was effective on the surface morphology of the prepared silicas, the morphological differences (f) with fewer aggregates confirm the composition analysis (XRF) discussed earlier, in which pre-treatment with L-cysteine significantly improved silica formation for the acid-leached sample. The acid treatment during the synthesis stage causes particle agglomeration, which is more obvious in the SEM image (c). After TPAH treatment, the SEM picture of the silica sample is clearer, showing that activated carbon lowered the degree of alkalinity for silica, resulting in less agglomeration [13].
3.2.4 Textural analysis
SCBA for nano CA-Silica had BETSSA, pore volume, and pore diameter of 21.6511 m2/g, 0.04312 cm3, and 8 nm, respectively, while SCBA for nano L-cys Silica had BETSSA, pore volume, and pore diameter of 116.005 m2/g, 0.1828 cm3/g, and 6 nm, respectively. The amount of adsorbed gas grows steadily with increasing P/P0 ratio at the lower P/P0 areas for CA-Silica, as shown in Figure 5a, whereas it increases dramatically for L-Cys Silica, as shown in Figure 5b. As mentioned in, this is commonly attributed to monolayer and multilayer adsorption [14].

Figure 5.
N2 adsorption-desorption isotherms for SCBA nano CA-Silica and SCBA nano L-cys silica that is made from SCBA, respectively. Adapted with permission from [
The hysteresis loop is detected at a range of 0.9 < P/P0 < 1.0 for the SCBA nanosilica isotherm in Figure 5a, which is primarily connected with capillary condensation taking place in the mesopores. Furthermore, the loop on the high P/P0 side has been linked to big pores. The little loop in Figure 5b, detected in a range of 0.2 < P/P0 < 0.4, is also caused by capillary condensation within the mesoporous structure; however, no loop is observed at the higher P/P0 area for this synthesized silica (SCBA nano L-cys Silica) [14, 15].
4. Recommendation
As an alternative to SCBA pretreatment, leaching with an organic acid, specifically citric acid (CA, C6H8O7). This organic acid has the potential to act as both a chelating agent and a mild triprotic acid. CA has the advantage of being an organic and bio-based acid, which has an impact on the materials synthesized and the effluent treatment costs, and it is an eco-friendly leachate when compared to strong mineral acids like HCl and H2SO4. Rodrguez-Machn et al. investigated the effect of leaching using CA for the pretreatment of SCBA with varying degrees of thermal degradation and its behavior. As shown in Table 2, CA was compared to two well-known conventional leaching agents, HCl and H2SO4, in terms of their impact on the chemical, structural, and thermal properties of leached ash. Inorganic element removal ranged from 39.9% to 54.1% depending on leaching duration and temperature, and CA performed similarly to strong mineral acids. When compared to traditional acids for the pretreatment of SCBA, this study shows that CA is a functional leaching agent [16].
| Element | Pristine SCBA | |||
|---|---|---|---|---|
| K | 1800 | 17.5 | 15.7 | 17 |
| Al | 279 | 110 | 122 | 131 |
| Fe | 327 | 110 | 101 | 123 |
| Si | 8600 | 3600 | 4400 | 4400 |
| Mg | 287 | 25.1 | 26.4 | 68.1 |
| Na | 32.1 | 18.6 | 8.02 | <5 |
Table 2.
Comparison between conventional strong acids (HCl and H2SO4) and an organic acid (CA, C6H8O7). Inorganic element composition in mg kg−1.
Table 3 illustrates the pre-treatment efficiency of HCl and demonstrates significant increase in the silica content and appreciable decrease impurities. To obtain silica with high purity metal oxides which are present as impurities defeats this goal to a certain extent. Thus, purification strategies are required to improve purity, such as thermochemical processes [17, 18].
| Component | Bagasse ash (Pristine) (wt%) | Bagasse ash (acid-treated) (wt%) |
|---|---|---|
| SiO2 | 53.10 | 88.13 |
| MgO | 20.72 | 3.04 |
| P2O5 | 7.36 | 1.15 |
| SO3 | 11.23 | 4.69 |
| MnO | 1.45 | 0.62 |
| Fe2O3 | 0.78 | 0.94 |
| K2O | 1.26 | 0.50 |
| CaO | 3.77 | 0.57 |
| Other | 0.36 | 0.32 |
Table 3.
SCBA treated with HCl, before and after [17].
Organic chemicals and less energy can be used to create green silica nanoparticles, allowing for fine control of particle form, size, and morphology. As a result, new innovative tactics and extra research have been developed to improve existing green technologies and methodology for future silica preparation. Toxic chemicals are utilized in the production of silica from SCBA, which is exceedingly dangerous to both the environment and humans. To begin, 1 M NaOH is used to extract silica in a water bath heated to 90°C, as given in Eq. (4), traditionally known as alkaline treatment. The sample is then filtered and decant supernatant several times until the pH is neutral, and it is dried in an oven. The as-obtained sodium silicate is then immersed in a strong acid 1 M HCl for neutralization reaction in a reflux for an hour at 75°C to make silica gel from sugarcane bagasse, as given in Eq. (5) [12, 19].
Greener and more sustainable synthetic technologies with eco-friendliness, low cost, low energy, low or non-toxicity, and easy procedures are extremely promising and should be prioritized by researchers. The utilization of proteins in the synthesis of multifunctional silica nanoparticles has important economic and environmental implications. To minimize high temperatures, energy, pressure, and the use of toxic and/or hazardous substances and circumstances, sustainable and eco-friendly silicon nanoparticle production technologies with enticing advantages over conventional methods are being developed. Organic acids and bases were used in this study to fulfill the green chemistry goal.
5. Conclusion
The green synthesis of silica nanoparticles from biomass waste (bagasse) is the basis of this research. The use of toxic-free reagents and substances in the green chemistry method for the synthetic route of nanoparticles is gaining traction. When compared to traditional approaches, this approach is seen as more environmentally friendly. It is critical to recognize the non-toxicity of nanoparticles produced using green chemistry. Green chemistry is less expensive, non-toxic, and better for the environment.
The silica nanoparticles were effectively manufactured utilizing the green synthesis approach. Meanwhile, the XRD spectra revealed the primary peaks of silica 2 = 28°, whilst the bands at 1045.99 cm−1 on the FTIR spectrum suggested silica production in the samples. Furthermore, approximately 80% of the SiO2 was removed utilizing the organic acid/base treatment method on the SCBA. The particle size distribution ranged from 25 to 500 nm. The synthesized nanomaterials revealed residue of impurities associated commonly with the green method.
Acknowledgments
This research was funded by National Research Foundation: 138079 and Eskom (South Africa): 2002/015527/0. This research work was supported by the University of the Western Cape and the Sustainable and Renewable Energy Nanomaterials research group.
References
- 1.
Duque-Acevedo M, Belmonte-Ureña LJ, Cortés-García FJ, Camacho-Ferre F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Global Ecology and Conservation. 2020; 22 :00902 - 2.
Shafey AME. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Processing and Synthesis. 2020; 9 (1):304-339 - 3.
Danevad D, Carlos-Pinedo S. Exploring interactions between fruit and vegetable production in a greenhouse and an anaerobic digestion plant—Environmental implications. Frontiers in Sustainability. 2021; 2 :111 - 4.
Mazari SA, Ali E, Abro R, Khan FSA, Ahmed I, Ahmed M, et al. Future challenges-a review. Journal of Environmental Chemical Engineering. 2020; 2020 :105028. DOI: 10.1016/j.jece.2021 - 5.
Rodríguez-Félix F, Graciano-Verdugo AZ, Moreno-Vásquez MJ, Lagarda-Díaz I, Barreras-Urbina CG, Armenta-Villegas L, et al. Trends in sustainable green synthesis of silver nanoparticles using agri-food waste extracts and their applications in health. Journal of Nanomaterials. 2022; 2022 :1-37 - 6.
Zuliani A, Rapisarda M, Chelazzi D, Baglioni P, Rizzarelli P. Synthesis, characterization, and soil burial degradation of biobased polyurethanes. Polymers. 2022; 14 (22):4948 - 7.
Karthik KV, Raghu AV, Reddy KR, Ravishankar R, Sangeeta M, Shetti NP, et al. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere. 2022; 287 :132081 - 8.
Rodríguez-Félix F, Corte-Tarazón JA, Rochín-Wong S, Fernández-Quiroz JD, Garzón-García AM, Santos-Sauceda I, et al. Physicochemical, structural, mechanical and antioxidant properties of zein films incorporated with no-ultrafiltered and ultrafiltered betalains extract from the beetroot (Beta vulgaris) bagasse with potential application as active food packaging. Journal of Food Engineering. 2022; 334 :111153 - 9.
Seroka NS, Taziwa R, Khotseng L. Green synthesis of crystalline silica from sugarcane bagasse ash: Physico-chemical properties. Nanomaterials. 2022; 12 (13):2184 - 10.
Kalapathy U, Proctor A, Shultz J. Production and properties of flexible sodium silicate films from rice hull ash silica. Bioresource Technology. 2000; 72 (2):99-106 - 11.
Katare VD, Madurwar MV. Experimental characterization of sugarcane biomass ash–a review. Construction and Building Materials. 2017; 152 :1-15 - 12.
Seroka NS, Taziwa RT, Khotseng L. Extraction and synthesis of silicon nanoparticles (SiNPs) from sugarcane bagasse ash: A mini-review. Applied Sciences. 2022; 12 (5):2310 - 13.
Carmona VB, Oliveira RM, Silva WTL, Mattoso LHC, Marconcini JM. Nanosilica from rice husk: Extraction and characterization. Industrial Crops and Products. 2013; 43 :291-296 - 14.
Shen L, Guo X, Fang X, Wang Z, Chen L. Magnesiothermically reduced diatomaceous earth as a porous silicon anode material for lithium ion batteries. Journal of Power Sources. 2012; 213 :229-232 - 15.
Li M, Dai Y, Ma W, Yang B, Chu Q.Review of new technology for preparing crystalline Silicon solar cell materials by metallurgical method. In: IOP Conference Series: Earth and Environmental Science. Vol. 94, No. 1. Bristol, United Kingdom: IOP Publishing; 2017. p. 012016 - 16.
Rodríguez-Machín L, Arteaga-Pérez LE, Pérez-Bermúdez RA, Casas-Ledón Y, Prins W, Ronsse F. Effect of citric acid leaching on the demineralization and thermal degradation behavior of sugarcane trash and bagasse. Biomass and Bioenergy. 2018; 108 :371-380 - 17.
Norsuraya S, Fazlena H, Norhasyimi R. Sugarcane bagasse as a renewable source of silica to synthesize Santa Barbara Amorphous-15 (SBA-15). Procedia Engineering. 2016; 148 :839-846 - 18.
September LA, Kheswa N, Seroka NS, Khotseng L. Green synthesis of silica and silicon from agricultural residue sugarcane bagasse ash–a mini review. RSC Advances. 2023; 13 (2):1370-1380 - 19.
Thuc CNH, Thuc HH. Synthesis of silica nanoparticles from Vietnamese rice husk by sol–gel method. Nanoscale Research Letters. 2013; 8 :1-10