Advantages and disadvantages of different gasifier.
Abstract
Due to the increasing demand for petroleum use as fuel, there has been a focus on the production of fuel that has a huge possibility for long-term energy sustainability. Synthetic gas (Syngas) is generated by means of a thermochemical technique known as gasification, which converts carbonaceous feedstocks (biomass, crude oil residuum, municipal waste, petroleum, and coal) to syngas. It contains carbon monoxide and hydrogen as the key elements of inflammable gas. It is widely used for gas lighting in coal gasification method before availability of electric lighting, gas turbine fuel, raw material for liquid fuels and the synthetic natural gas, and anode gas of solid oxide fuel cells. It is synthesized either through the gasification of plant-based biomass or pyrolysis of waste. This chapter will focus on the information, which has been rounded up over the last decades on syngas properties, sources, and production at a competitive advantage, as well as application and future technological advancement.
Keywords
- synthetic gas
- gasification
- pyrolysis
- thermochemical process
- petroleum product
- sustainable energy source
1. Introduction
Syngas is also known as the “synthesis gas” and producer gas that could be synthesized from many kinds of resources, which consist of carbon. Energy resources such as biomass, coal, plastics, municipal waste, and other comparable materials. Synthesis gas consists of a mixture of H2, CO, and CO2 that could be utilized as a potential intermediate in the transformation of biomass into fuel. Raw syngas also carries mostly notable amounts of CO2 and H2O. It is also a short name used for a gasification product, mainly from waste biomass. All of the chemicals and goods that the petrochemical sector today produces are constructed from syngas. Possibly, decisive and the demanding applications of the synthesis gas as precursor for the liquid fuel.
Biomass is a CO2 neutral option for energy production, and the potential for the conversion of biomass into energy is expanding quickly [1]. According to Maniatis, energy from biomass based on short rotation forestry (SRF) and other energy crops performed valuable contribution toward achieving the objectives of the Kyoto agreement in reducing the greenhouse gas emissions and the problems related to climate change [2]. Furthermore, many biomass technologies are present for conversion of biomass to energy. These technologies can change raw biomass into a variety of gaseous, liquid, or solid materials that can then be used for the generation of energy.
This conversion can be performed in three ways: thermochemical (break down of biomass at high temperature), biochemical (break down of biomass in the presence of microorganism or enzymatic processes), and chemical (oils from biomass can be chemically converted into a liquid fuel) [3]. Over the last few years, there have been many developments in the science and technology of thermochemical biomass conversions. Incineration, gasification, and pyrolysis conversion are among the established and best available thermochemical technologies [2, 3]. These thermal processes provide sustainable energy sources that are efficient, environmentally acceptable, and cost-effective. Gasification is the thermochemical conversion of a solid biomass to a gaseous fuel through heating in the presence of gasification agent (i.e., air, oxygen, steam, hydrogen, CO2, or mixtures of these gases).
It is the technological technique, which could convert carbonaceous raw materials such as biomass, coal, and waste in fuel gas that also called as syngas. Syngas got its name from its usage as in-between in synthesis of the SNG (synthetic natural gas). Primarily, the syngas is utilized in synthesis of other chemicals and fuels such as methanol and diesel fuel at higher pressures [4]. Generally, syngas might indicate combination of (i) nitrogen/hydrogen (N2/H2), which is utilized to produce ammonia, (ii) carbon monoxide and hydrogen for the production of synthetic hydrocarbons in the gas-to-liquids plants along with methanol and ethanol in the petrochemicals [5], or (iii) carbon dioxide and hydrogen (CO2/H2) to produce hydrogen [6, 7].
Synthesis gas is the significant component of chemical processing industries, and the hydrogen has been a major part of the syngas. The composition of the syngas has highly dependent on the raw material and manufacturing technique but can be thought of as mixture of 30–50% carbon monoxide (CO), 25–30% hydrogen (H), 5–15% carbon dioxide (CO2), and 0–5% methane (CH4). Syngas produces diesel fuel by the Fischer-Tropsch technique. This method turns combination of hydrogen and carbon monoxide into liquid hydrocarbons by series of reactions. These chemical reactions could occur in existence of metal catalysts at the temperatures, ranging from 150°C to 300°C (302°F–572°F).
Syngas is a fundamental intermediate product all over the chemical industry. Each year, almost 6 EJ of syngas is synthesized throughout the world, which is nearly 2% of the main energy consumption of the world. The global syngas market is dominated by the ammonia industry (largely from the fossil fuels e.g., natural gas, coal, oil, and residues) [7]. There are various applications of syngas such as the synthesis of the hydrogen for usage in the refineries, such as processing of the hydrogen, gas-turbine fuel, anode gas of the solid oxide fuel cells, the raw material for synthetic natural gas (SNG), and methanol synthesis and liquid fuels. Frequently, these applications need progressions for cleaning synthesis gas to remove impurities that arise from the coal, which comprise sulfur compounds, mercury, hydrogen halides, alkali metals, and trace elements [8].
2. Numerous feedstocks as energy source
For eras, coal had been used as prime feedstock for the gasification process then due to the current concern regarding its usage as fossil fuels, and subsequent environmental contaminants, there is interchange to other feedstocks except the coal [9]. In the gasification process, carbonous feedstocks, which would be unused and cast-off, for example, waste biomass can be used as energy sources to produce syngas. Several feedstocks, for example, biomass, other carbonaceous wastes, and crude oil residues could be utilized to their maximum potential [10].
The gasification process could take different feedstocks, but the reactor that used in the gasification process must be chosen on the base of behavior and feedstock properties in the process. When various feedstocks (carbonaceous or hydrocarbonaceous) have been employed in gasification than product (such as syngas) has possibly been more suitable as the energy resource as compared to other feedstocks. Syngas production has been more effective energy source than the direct combustion of feedstock because the syngas can be combusted on the high temperature, utilized in fuel cell, use to produced methanol, used as hydrogen source, and converted into range of the synthesis liquid fuels that are fit to use for diesel engines, gasoline engines, or for the production of wax [11] (Figure 1).
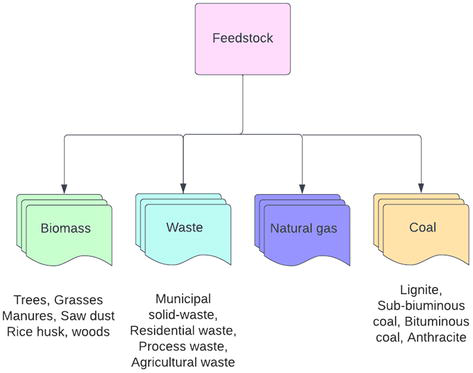
Figure 1.
Different feedstock as energy sources.
2.1 Natural gas
Searching for the alternate energy resources to substitute the petroleum fuels, the natural gas is engrossed attention of numerous scientists and huge quantity of the methane comprised in the natural gas is contemplated as the contribution in the production of high-valued products, for example, synthesis gas and the great purified hydrogen. Although synthesis gas could be generated from feedstock diversity, for example, petroleum, coke, coal, natural gas, and biomass, but lowest- cost routes to produce syngas have been based on the natural gas [12].
Natural gas demand as energy source has been increased gradually. Due to lack of hetero atoms and high H:C ratio make the natural gas as key feedstock for the synthesis fuel, which could swap these which have been characteristically petroleum-derived products. Additionally, novel arenas of gas and oil comprise 8–18% of associated CO2, and in approximately precise field, allied gas comprises of high CO2 (such as 79%). In this situation, process such as tri-reforming syndicates two problematic gases of greenhouse (CO2 or CH4) to produce synthetic gas for mixture of the cleaned liquid fuel and valued chemicals [13].
Assuming the up-to-date level of the consumption of the natural gas for the world has been upheld, standby should be adequate to last for 64 years. Though, in assessment of the natural gas permanency, influences, for example, upsurge in the annual consumption, finding of novel reservoir, the improvements in the discovery technologies, and the use of the hydrates of natural gas have not been involved. So, the outcome of gas discovery in fitted a slate formation that is balanced over annual consumption due to the discoveries of major fields of natural gas the world’s natural gas reserves are generally in an upward trend [14].
2.2 Coal
Chemically, coal is the hydrogen lacking hydrocarbon having an atomic H:C ratio close to 0.8, for example, compared to hydrocarbon derivatives of crude oil, which have an atomic H:C ratio almost equivalent to the 2, and the methane, which has atomic H:C ratio equivalent to the 4. Thus, any process that has been utilized to change the coal into alternate fuel should add hydrogen in natural coal to make coke and ionic product of hydrogen [15].
Various coal types in the organic sedimentary rock, which has been formed from the preservation and accumulation of the plant materials, generally in the marsh environment [7, 8]. It is flammable rock, and laterally with natural gas and oil it has made one of three significant fossil fuels for production of the electrical energy and affords about 40% of the electrical energy production on global basis. It has been studied widely for the conversion into liquid and gaseous along with hydrocarbon feedstocks. Due to his comparative profusion and the constant fuel prices on the market, coal is an important objective for the synthetic adaptation in other forms of fuel, for example, synfuels. It can be gasified along with the additional fuels containing oil, biomass, scrap tires, and the municipal wastes [7, 9, 16, 17, 18]. Coal gasification as power-generation technology has been gaining popularity because of global accessibility of coal raw material, in addition to positive environmental issues related to that technology over the other combustion technologies.
Hence, the gasification process provides a versatile and cleanest method to change energy in hydrogen, electrical energy, and other sources of power that contained in coal. Turning of coal in syngas has not been a new idea; actually, the coal gasification method times following prior to World War II. The gasification could process effectively those entire residues and the wastes which have been formed in the processing planted to the improved production of the inflated valued products by the advancement of their crude feedstock [14].
2.3 Biomass
Biomass is the renewable source, which has been established significant consideration due to the conservational contemplations and growing energy demands worldwide [19, 20]. Biomass has been generated through a process called photosynthesis, which includes chemical reactions that occur on the Earth among green plants and sunlight in chemical energy form. For instance,
There are several forms of the biomass, which could be utilized and replace deprived of irrevocably reducing assets, and usage of the biomass would endure to raise in standing as substitute for sources of fossil fuel and the feedstocks for variety of the products [16, 18, 19]. Up to 35% of the energy needs of emerging nations are met by biomass, which is also equivalent to 13% of the global energy demand. Biomass has been broadly obtainable in the amounts that are adequate to meet world energy demand [21, 22].
Gasification of the biomass resources is the most attractive method to produce a gas that is highly rich in hydrogen. Production of syngas from the biomass has been the most suitable solution for the energy disaster. Depletion of fossil fuel energy in the developing countries can be overcome by utilizing biomass as energy source, for example, ample biomass has been obtainable in the developing countries, and it also renewable [21, 23]. Gasification signifies as effective and the environment friendly technique to produce syngas as biofuel from diverse biomass resources [24] and generate second biofuel generation, for example, hydrogen, ethanol, and methanol [25]. Gasification could be definite as partial biomass combustion, and it can extract till 60–90% of the energy that deposited in biomass [26]. Worldwide, biomass energy is generally integrated in power production system such as the United States commenced incomplete and complete conversion of the traditional power stations to the biomass [27].
2.4 Waste
Focus on the modern management of waste has been considering shift to the production of energy, although handling sustainably of waste. Subsequently, the treatment of waste by gasification has gradually attained waste burning with numerous aids, comprising instantaneous waste management and the production of energy, although dropping landfill volume and dislocating conservative fossil fuels. Just 3% of the entire solid waste has been utilized to produce energy; there is significant capability to utilize the residual waste for the recovery of energy [28].
Gasification of the waste feedstocks is not different to gasification of biomass because they are also hydrocarbons that are used to produce synthesis gas. Waste gasification has been favored over the burning as it provided synthesis gas product, which could be utilized in several applications as contrasting to the hot gases of incineration. Waste gasification provides the even quality synthesis gas from mixed and multifaceted residual waste. It is the solitary option, which could provide multimodal products, for example, heat, cooling, power, liquid, and gaseous fuels in addition to chemicals [29].
3. Production of syngas
3.1 Gasification of biomass
Biomass gasification has one of the attractive approaches to produce gas that are highly rich of hydrogen. It converted carbonous biomass to gases such as hydrogen, carbon monoxide, and carbon dioxide [30]. This method could be attained through a chemical reaction of the feed at 700°C, with the inadequate volume of stream and oxygen. In gasification of biomass, feed has been treated with some portion of combustion to maintain the high gasification reaction temperature. In this process, engendered mixture of the gas has been considered as synthetic gas and producer gas used as fuel [31]. The power produced in gasification of biomass and generating gas combustion could be considered as the renewable resource of energy.
The reactions of biomass gasification have been taken place in gasifier that could be concise as indicated below [23, 32, 33]:
Partially oxidation:
Complete oxidation:
Water-gas phase reaction:
Boudouard reaction:
Heat requires for the water gas stage and the Boudouard reactions have been performed through partial and complete oxidation reactions, and the complete oxidation reaction provides about 60% of heat necessities in gasification [23].
The biomass gasification has been the probable resource to produce biofuels and energy, especially chemical energy. The gasifier converts renewable energy resources of biomass to the syngas in the gasification process. Produced syngas has been utilized to function of the internal combustion engine. Syngas could be utilized to generate heat energy and electricity through co-generation system. Almost, biomass energy resources are requiring drying first. Later, dry materials have been required for the process of devolatilization [34].
There are following advantages of biomass gasification: (1) Produces a more practical, readily controllable kind of cleaner fuel for the production of thermal energy, as well as electricity, and offers a way to cut back on or dip away with traditional fossil fuels. (2) Gasification enables biomass to be used as a fuel for a variety of power generation technologies, including gas turbines, fuel cells, and reciprocating engines. (3) It is possible to gasify a wide range of biomass materials, many of which would be challenging to burn otherwise. (4) Gasification has the ability to lower emissions of greenhouse gases and other pollutants per unit of energy produced [30].
3.2 Gasification of waste
Gasification of waste has been chosen over the incineration because it provides different syngas product that utilized in numerous forms while contrasting to the hot gases of combustion. It provides the even quality synthetic gas from the varied and composite waste residues. The gasification of waste is lone option that could offer multimodal products, for example, cooling, heat, power, liquid, and gaseous fuels along with chemicals [29].
It comprises four different phases, which are following such as feedstock drying, pyrolysis, oxidation, and the reduction [35].
3.2.1 Drying
Waste feedstocks having variable moisture level have been dry in the drying process beyond 100°C. In that stage, chemical reactions do not take place, the phase alter among liquid to water and the vapor that is core cause of energy requirements in the drying procedure.
3.2.2 Pyrolysis
In pyrolysis, feedstock decomposition starts in the lack of the oxidant on high temperature, and the vapors have been free from dried feedstock by primary reactions. Proportion of vapors and the produced char are predisposed through the conditions of process, for example, temperature and heating rate. In addition, product distribution has been affected by the composition and size of feedstock [36].
3.2.3 Oxidation
On high temperature and in the environment that is partially oxidized, the heterogeneous reactions occur among CO, oxidant, and water vapor of feedstock. Oxidation reaction has influenced through chemically feedstock composition, oxidant types (such as oxygen, CO2, air, or steam,), and the operating conditions. Generally, oxidation phase is an exothermic phase, and heat energy releases as a result of it for energy independence to endure heating requirements of the process.
3.2.4 Reduction
It is an endothermic phase through which the high-temperature chemical reaction takes place in oxygen absence. Several reactions among products of oxidation and the char occur to produce novel hydrocarbons. Some char and ash are the by-products of reduction phase [35, 37].
Advantages of gasification of waste are: waste is gasified, which minimizes the demand for landfill space, lowers methane emissions, and uses fewer fossil fuels. Thus, waste gasification is employed to improve recycling initiatives [36].
3.3 Gasification of coal
Coal gasification is a method in which coal, char, or coke is converted into gassy fuels through contact with the mediators, for example, steam, oxygen, or air. While a certain gas amount has been freed from coal through carbonization methods (such as heating of in nonexistence of oxygen or air), and complete coal gasification comprises the conversions of all carbonous material to the gassy products through reaction that are given below:
3.3.1 Primary reactions
Water gas reaction:
Boudouard reaction:
Partial combustion:
Hydrogasification:
3.3.2 Secondary reactions
Shift reaction:
Methanation:
The only residues of the complete coal gasification would, hence, be ash-forming components of mineral matter that are released solid form (slag) [38]. Depending on coal nature and actual gasification method, products of the gasification might include CO2, CO, CH4, and H2 diluted
Synthesis gas, combination of CO and H2, with 9 kJ m−3 specific energy.
Synthetic natural gas, essentially made of methane, with 30 kJ m−3 specific energy.
Syngas could be utilized to produce amount of products, containing methanol, ammonia, and liquid fuels, but the synthetic natural gas is utilized as substitute for the natural gas in industrial and domestic reticulation schemes [39].
Gasifiers are used in coal gasification and are fundamentally bounded pressure vessel in which pulverized or crushed coals has been interacted with the gasifying agent. Lurgi fixed bed gasifier with the jacketed vessel of water having diameter of 4 m is one of the widely used gasifiers for coal gasification. Indelicately, the crushed coal has been fed in by lock hopper structure at the top and passes downward slowly alongside upward flow of steam and oxygen. Products of hot gasification have been removed from the top, whereas the ash has been removed innextlock arrangement on base.
A more eco-friendly and less-polluting way to treat coal is through coal gasification. When burned, it releases less carbon, and other harmful gases, such as CO2, are simple to separate, capture, and utilize in other ways. Coal gasification syngas can also be further processed to produce fuels such as petrol and diesel. Other coal gasification by-products, such as hydrogen, can be employed in the production of ammonia, the search for alternative fuel sources, or the petrochemical sector [38].
3.4 Gasification of natural gas
Partial oxidation (POx) is also known as gasification. By using natural feedstock, we use partial oxidation as gasification process to produce syngas. Chemistry of partial oxidation process has been founded on the partial fuel combustion, such as in CH4 case, that is represented in the following equation
Though, this process of partial oxidation is mostly utilized to produce syngas from the heavy hydrocarbons, with petroleum coke and deasphalted pitch. After ignition, feedstock, such as natural gas, is preheated and then mixed with the oxygen inside burner; reaction occurred inside the elevated temperature combustion chamber produce effluent, which comprise of numerous quantities of soot, depending on composition of feedstock.
Typically, exodus gas temperature of reactors is included among 1200–1400°C. Attained synthesis gas must cool and cleaned inside “washing” sector to remove impurities. Elevated temperature (such as 1400–1100°C),the recovery of heat in partial oxidation has not been very effectual, and POx benefit over stream reforming is in option of utilizing the “low-worth” feedstocks and comprising sulfur and the other numerous compounds, which are fatal to catalysts of stream reforming. Presently, key utilizations of the partial oxidation are: (1) in the H2 production for the refinery applications, (2) production of syngas from natural gas and (3) in electricity production from deasphalted bottoms and petroleum coke, by using large integrated gasification combined cycles (IGCC) [40].
3.5 Gasification using different gasifiers
It is the complicated process that has been influenced by numerous factors among which design of the equipment plays a significant role. Some important and popular gasifiers have been listed and briefly discoursed as bellow.
3.6 Fixed bed gasifier
It is a type of gasifier in which there are three ways to enter the gasifying agents in reactor to react with each other such as downdraft, updraft, and cross-draft gasifier. Updraft gasifier is explained below.
Updraft gasifier is also known as countercurrent gasifier in which air/oxygen has been passed through bottom level of gasifier, and produced product gases have been left at the top of gasifier [41]. The combustion reactions have been occurring at the bottom of gasifier near grate. After combustion, reduction reactions have been occurring at slightly upper level of the combustion zone, as displayed in Figure 2. In the upper level of the gasifier, pyrolysis and heating of feedstock materials have been occurred consuming forced convection and the radiation heat transfer method, wherever required heat has been provided from the reduction and combustion zone of gasifier [43]. The produced tars and volatile matters in updraft gasifier have been carried in upper-level gas stream, as showed in Figure 2. Alternatively, produced ash has been required to clean in bottom of updraft gasifier.
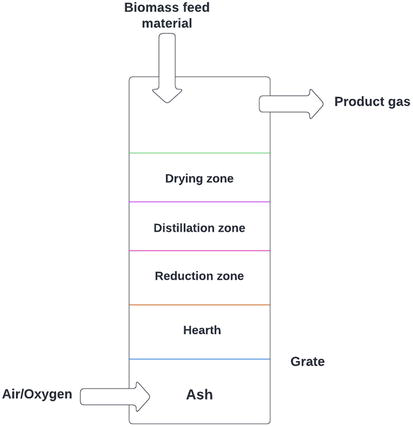
Figure 2.
Gasification process using fixed-bed (downdraft) gasifier [
3.7 Fluidized bed gasifier
In fluidized bed gasifier, the fuel has been fluidized with air and steam. The fuel has been fed into circulating or bubbling form of fluidized bed. In fluidized bed gasifier, bed acts as fluid having high turmoil. In this gasifier, ash has been removed from gasifier in the dry state, which de-fluidize. In fluidized bed gasifier, the temperature is low, and highly reactive fuel is required [44]. Though, energy conversion efficacy has been lower than fixed bed gasifier due to elutriation of the carbonaceous fuel [45]. Fluidized bed gasifier is of three types such as bubbling, circulating, and dual fluidized bed.
The principle of downdraft and updraft and gasifier has been affected by the physical and chemical properties of fuel. Fluidized bed gasifiers could solve few problems of fixed bed gasifiers, for example, low bunker flow and pressure drop over gasifier. It has ability to tolerate high distinction of the fuel quality along with large particle distribution [46].
3.8 Entrained flow gasifier
In entrained flow gasifier, the liquid fuel, dried solid milled, or slurry has been reacted with air/oxygen in the gasification process through cocurrent flow. In this gasifier, the gasification reactions have been taken in denser cloud of the fine particles. In which high output could be attained, but overall efficacy is comparatively low than fixed-bed and fluidized bed gasifier. The residence time of the entrained flow gasifier is about 5 seconds, which has been shorter than residence time of fixed-bed and fluidized bed gasifier. In this type of gasifier, most of the reactions are endothermic. So, high heat has been required that is supplied through the combustion of feed material and from external sources of the heat [47].
In this type of gasifier, fine amount of coal with the air has been added co-currently, so air and steam surround coal feedstocks. Usually, it works at high temperature and pressure [48]. Therefore, flow is turbulent in entrained flow gasifier. Rate of the gasification reaction and efficacy of carbon conversion is high, although production of hydrocarbons has been low. Furthermore, coal devolatilization procedure produces oil, tar, phenol, and other liquids, which could be decomposed into hydrogen (Table 1).
| Gasifier | Advantages | Disadvantages |
|---|---|---|
| Updraft fixed bed |
|
|
| Downdraft fixed bed |
|
|
| Fluidized bed |
|
|
| Entrained flow |
|
|
Table 1.
4. Applications
4.1 Gaslighting
Before, widely availability of natural gas and electric lighting, syngas was utilized for the synthesis of illuminating gas (such as coal gas) for many years. Illuminating gas used for cooking, gaslighting, and heating. Gaslighting has been a process in which artificial light production takes place through the combustion of gaseous fuel, for example, hydrogen, carbon monoxide, methane, propane, acetylene, ethylene, butane, coal gas, and natural gas, etc. Light has been generated directly from flame, to enhance the brightness of illuminating light special combination of propane or butane has been used.
4.2 Energy capacity
Unmethanized syngas generally has a lower heating value, that is. Nearly equal to 120 BTU/scf. Hybrid turbines could be proceeding by using untreated syngas, which allows for higher efficacy due to their lower operating temperature and extended part lifetime. The output power of synthesis gas-fired turbine plant can be enhanced by up to 20–25% in comparison to the similar turbine fired at the same operating temperatures as the natural gas. Nevertheless, rise in the power output has been attributed to increase in moisture level of products of combustion. Due to the greater amount of hydrogen in syngas and amplified turbine flow, both of which make a significant contribution toward turbine component overheating [49].
4.3 Diesel
Synthesis gas could be utilized in Fischer-Tropsch technique for the production of diesel. It also used to convert syngas in methane, dimethyl ether, and methanol in the catalytic processes. Mostly, methanol has been synthesized from synthesis gas. Although most of the methanol synthesis has been based on the natural gas as feedstock, coal-derived synthesis gas also used; as solid/coal, feedstocks have been utilized to produce 9% of global yield of the methanol [50].
4.4 Hydrogen production
Syngas has been used to synthesize hydrogen by the Haber process. The synthesis gas generated because of the gasification procedure comprises hydrogen and carbon monoxide, which is again reacted with the steam to separate the hydrogen.
4.5 Power generation from syngas
The primary application of syngas is generally the generation of power and heat. This can be achieved either in autonomous combined heat and the power plants or by cogeneration of gas produced in large-scale plants. Synthetic gas from pyrolysis is a fuel gas that can be used to produce electricity in a wide range of equipment, from steam cycles to gas engines and turbines. Whereas, boiler applications for steam cycles generally do not require intensive gas treatment prior to electricity production. Gas engines require more advanced purification and processing.
The stability and consistency of fuel provided to the internal combustion engine are one of the important elements of the technique. It is guaranteed by stability of feedstock and the precisely controlled processing conditions in the bio green pyrolysis unit [51]. Flow sheet of different applications of syngas is illustrated in Figure 3.
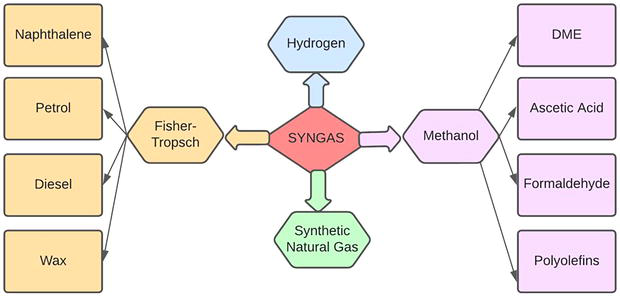
Figure 3.
Applications of syngas [
5. Conclusion
Synthesis gas is definite as gas with CO and H2 as the key components of fuel. Gasification is one of attractive approaches to produce hydrogen-rich gas. Synthesis gas production with improved properties from different feedstocks (such as biomass, waste, coal, and natural gas) has been an attractive solution for the energy crisis. Syngas through gasification has been used in different applications to overcome the energy crises of petroleum products.
Acknowledgments
The authors acknowledge faculty of life sciences and vice chancellor for any kind of assistance provided by them.
References
- 1.
Ohlström M, Mäkinen T, Laurikko J. New concepts for biofuels in transportation biomass-based methanol production and reduced emissions in advanced vehicles. VTT Technical Research Centre of Finland. 2001; 2001 :3-94 - 2.
Maniatis K. In: Bridgewater AV, editor. Progress in Biomass Gasification: An Overview. Oxford: Blackwell Scientific Publications; 2001 - 3.
Faaij A. Modern biomass conversion technologies. Mitigation and Adaptation Strategies for Global Change. 2006; 11 :343-375 - 4.
Capodaglio AG, Olsson G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the urban water cycle. Sustainability. 2020; 12 (1):266. DOI: 10.3390/su12010266 - 5.
Martin MM. Industrial Chemical Process Analysis and Design. Cambridge, MA: Elsevier; 2016 - 6.
Salim W, Ho WSW. Hydrogen purification with CO2-selective facilitated transport membranes. Current Opinion in Chemical Engineering. 2018; 21 :96-102 - 7.
Voldsund M, Jordal K, Anantharaman R. Hydrogen production with CO2 capture. International Journal of Hydrogen Energy. 2016; 41 (9):4969-4992 - 8.
Díaz JA, Akhavan H, Romero A, Garcia-Minguillan AM, Romero R, Giroir-Fendler A. Cobalt and iron supported on carbon nanofibers as catalysts for Fischer-Tropsch synthesis. Fuel Processing Technology. 2014; 128 :417-424 - 9.
Kobayashi M. Chapter 1 – Introduction. In: Dry Syngas Purification Processes for Coal Gasification Systems. Elsevier; 2021. pp. 1-49. DOI: 10.1016/B978-0-12-818866-8.00001-X - 10.
Speight JG. The Chemistry and Technology of Petroleum. 5th ed. Boca Raton, Florida: CRC Press, Taylor & Francis Group; 2014 - 11.
Speight JG. Handbook of Petrochemical Processes. Boca Raton, Florida: CRC Press, Taylor & Francis Group; 2019 - 12.
Spath PL, Dayton DC. Preliminary Screening Technical and Economic Assessment of Synthesis Gas to Fuels and Chemicals with Emphasis on the Potential for Biomass Derived Syngas. Golden: National Renewable Energy Lab Golden Co.; 2003. DOI: 10.2172/1216404 - 13.
Roseno KT, Alves RMB, Giudici R, Schmal M. Syngas production using natural gas from the environmental point of view. Biofuels - State of Development. 2018; 2018 :97-113. DOI: 10.5772/intechopen.74487 - 14.
Speight JG. Shale Gas and Shale Oil Production Processes. Cambridge, MA: Elsevier Inc.; 2020 - 15.
Speight JG. Heavy and Extra Heavy Oil Upgrading Technologies. Oxford, United Kingdom: Gulf Professional Publishing, Elsevier; 2013 - 16.
Speight JG. Synthetic Fuels Handbook: Properties, Processes, and Performance. New York: McGraw-Hill; 2008 - 17.
Speight JG, editor. Biofuels Handbook. London, United Kingdom: Royal Society of Chemistry; 2011 - 18.
Speight JG. The Refinery of the Future. Oxford, United Kingdom: Gulf Professional Publishing, Elsevier; 2011 - 19.
Lee S, ShahYT. Biofuels and Bioenergy: Processes and Technologies. Boca Raton Florida: CRC Press, Taylor & Francis Group; 2013 - 20.
Hornung A. Transformation of Biomass: Theory to Practice. Chichester, West Sussex, United Kingdom: John Wiley & Sons Inc.; 2014 - 21.
Sheth PN, Babu B. Experimental studies on producer gas generation from wood waste in a downdraft biomass gasifier. Bioresource Technology. 2009; 100 :3127-3133 - 22.
Kirubakaran V, Sivaramakrishnan V, Nalini R, Sekar T, Premalatha M, Subramanian P. A review on gasification of biomass. Renew Sustainable Energy Review. 2009; 13 :179-186 - 23.
Zabaniotou AA, Skoulou VK, Mertzis DP, Koufodimos GS, Samaras ZC. Mobile gasification units for sustainable electricity production in rural areas: The smart-CHP project. Industrial and Engineering Chemistry Research. 2011; 50 :602-608 - 24.
Asadullah M. Barriers of commercial power generation using biomass gasification gas: A review. Renew Sustainable Energy Review. 2014; 29 :201-215 - 25.
Radwan AM, Hamad MA, Singedy AM. Synthesis gas production from catalytic gasification of saw dust. Life Science Journal. 2015; 12 :104-118 - 26.
Zhang W. Automotive fuels from biomass via gasification. Fuel Processing Technology. 2010; 91 :866-876 - 27.
Bhavanam A, Sastry R. Biomass gasification processes in downdraft fixed bed reactors: A review. International Journal of Chemical Engineering Applications. 2011; 2 :425-433 - 28.
Saghir M, Rehan M, Nizami AS. Recent Trends in Gasification Based Waste-to-Energy. Gasification for Low-grade Feedstock. Vol. 2018. London, UK: Intechopen; 2018. pp. 97-113 - 29.
Prins MJ, Ptasinski KJ, Janssen FJ. From coal to biomass gasification: Comparison of thermodynamic efficiency. Energy. 2007; 32 (7):1248-1259 - 30.
Baliban RC, Elia JA, Floudas CA. Toward novel hybrid biomass, coal, and natural gas processes for satisfying current transportation fuel demands, 1: Process alternatives, gasification modeling, process simulation, and economic analysis. Industrial & Engineering Chemistry Research. 2010; 49 (16):7343-7370 - 31.
Modell M, Reid RC, Amin SI. Gasification process. 1978. Google Patents - 32.
Buragohain B, Mahanta P, Moholkar VS. Biomass gasification for decentralized power generation: The Indian perspective. Renew Sustainable Energy Reviews. 2010; 14 :73-92 - 33.
Tavasoli A, Ahangari MG, Soni C, Dalai AK. Production of hydrogen and syngas via gasification of the corn and wheat dry distiller grains (DDGS) in a fixed-bed micro reactor. Fuel Processing Technology. 2009; 90 :472-482 - 34.
Das BK, Hoque S. Assessment of the potential of biomass gasification for electricity generation in Bangladesh. Journal of Renewable Energy. 2014; 2014 :10 - 35.
Demirbas A. Biomass gasification for power generation in Turkey. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2006; 28 (5):433-445 - 36.
Bridgwater AV. Renewable fuels and chemicals by thermal processing of biomass. Chemical Engineering Journal. 2003; 91 (2):87-102 - 37.
André RN. Fluidised bed co-gasification of coal and olive oil industry wastes. Fuel. 2005; 84 (12):1635-1644 - 38.
Chadwick MJ, Highton NH, Lindman N. Coal conversion technologies. In: Environmental Impacts of Coal Mining & Utilization. Stockholm, Sweden: Pergamon Press, Elsevier Inc.; 1987. pp. 129-130 - 39.
Ward CR. Coal Geology. Netherland: Elsevier Inc.; 2013. p. 29 - 40.
Laquaniello G, Iaquaniello G, Antonetti E, Cucchiella B, Palo E, Salladini A, et al. Natural Gas Catalytic Partial Oxidation: A Way to Syngas and Bulk Chemicals Production. London, UK: IntechOpen; 2012. DOI: 10.5772/48708 - 41.
Pedroso DT, Machín EB, Silveira JL, Nemoto Y. Experimental study of bottom feed updraft gasifier. Renewable Energy. 2013; 57 :311-316 - 42.
Phillips J. Different types of gasifiers and their integration with gas turbines. In: Proceedings of 1. World Conference and Exhibition on Biomass for Energy and Industry. 2006. p. 1 - 43.
Brandt P, Henriksen UB. Decomposition of tar in gas from updraft gasifier by thermal cracking. In: 1st World Conference and Exhibition on Biomass for Energy and Industry. USA: Natural Energy Technology Department; 2000 - 44.
Mansaray K, Ghaly A, Al-Taweel A, Hamdullahpur F, Ugursal V. Air gasification of rice husk in a dual distributor type fluidized bed gasifier. Biomass and Bioenergy. 1999; 17 (4):315-332 - 45.
Basu P. Combustion and Gasification in Fluidized Beds. Boca Raton: CRC Press; 2006 - 46.
Warnecke R. Gasification of biomass: Comparison of fixed bed and fluidized bed gasifier. Biomass and Bioenergy. 2000; 18 (6):489-497 - 47.
Wen CY, Chaung T. Entrainment coal gasification modeling. Industrial & Engineering Chemistry Process Design and Development. 1979; 18 (4):684-695 - 48.
Kajitani S, Suzuki N, Ashizawa M, Hara S. CO2 gasification rate analysis of coal char in entrained flow coal gasifier. Fuel. 2006; 85 (2):163-169 - 49.
Oluyede EO, Phillips JN. Fundamental impact of firing syngas in gas turbines. In: Proceedings of the ASME Turbo Expo 2007: Power for Land, Sea, and Air. Vol. 3. ASME; 2007. pp. 175-182. DOI: 10.1115/GT2007-27385 - 50.
State of the Gasification Industry. Worldwide Gasification Database 2014 Update Gasification Technologies Conference. Washington, DC: Chris Higman; 2014 - 51.
Chatterjee A. Sponge Iron Production by Direct Reduction of Iron Oxide. United States: PHI Learning; 2012. ISBN 978-81-203-4659-8. OCLC 1075942093 - 52.
Jie Y, Odriozola JA, Reina TR. Dry reforming of ethanol and glycerol: Mini-review. Journal of Catalysis. 2019; 9 :1015. DOI: 10.3390catal9121015