Comparative efficacy of imaging modalities in diagnosing neglected appendicitis.*
Abstract
Prompt diagnosis and surgical excision of inflamed appendix vermiformis is a goal that is often elusive in clinical practice. An overall delay more than 36 hours from the onset of symptoms can be defined as neglected appendicitis. Factors responsible for delay may be pre-hospital versus in-hospital, physician centered versus patient centered, medical versus socioeconomic and modifiable versus unmodifiable. Consequences of neglected diagnosis or treatment may be perforation, general peritonitis, adhesive bowel obstruction, prolonged ileus, mass formation, appendicular and metastatic abscesses, pyelephlebitis, stump appendicitis and fecal fistula. Non-operative antibiotic treatment followed by interval appendicectomy is recommended in neglected appendicitis to avoid collateral surgical injury to inflamed cecum. If surgical excision is unavoidable in acute phase, mucosa coring salvage (MUCOSAL) appendicectomy is preferred. It is important to recognize that clinical neglect is not the same as criminal neglect.
Keywords
- appendicitis
- appendicectomy
- acute abdomen
- delayed diagnosis
- neglected treatment
- medicolegal issues
- imaging studies
1. Introduction
Appendicitis is one of the most common surgical emergencies throughout the world [1, 2]. Its incidence is 100 per 100,000 person years [2]. Of the 675,000 children visiting emergency department (ED) for abdominal pain, about 80,000 (11%) underwent appendectomy [3]. It is now well established that prompt diagnosis and early surgical removal of the inflamed appendix is paramount for avoiding morbidity and mortality. However, this goal often remains elusive in clinical practice.
The diagnosis of appendicitis is missed in 4.8 to 15% of children and in 5 to 23% of adults during their first visit to ED [3, 4]. In some series the diagnostic error was as high as 53% [5]. Accurate differential diagnosis remains a challenge even in the era of modern imaging [3]. Despite routine use of CT scan and ultrasound the frequency of misdiagnosis persisted at 15% over 2 decades (Figure 1) [6, 7]. However, a few studies have noted a drop in misdiagnosis from 25–6% when a CT scan is used [8]. There are some indications that the rate of missed diagnoses is steadily falling in recent times, perhaps due to increased experience with modern imaging methods. The rate of missed diagnosis was 28% in 1990 [9], 7% in 1993 [10], and 4.8% in 2013 [3]. Even if a moderate diagnostic error of 5% is acknowledged, its cumulative impact will be huge. For example, in 2019, globally 17.7 million patients were newly diagnosed with appendicitis and by estimate 880,000 patients would have been misdiagnosed [11]. Consequences of diagnostic delay are deferred treatment, prolonged hospitalization, increased morbidity, higher mortality and undue escalation of the cost of health care [12].

Figure 1.
Graph showing the absence of any improvement in the misdiagnosis rate of acute appendicitis over a decade. (Constructed based on the data from: Flum DR, et al. JAMA 2001).
2. Definition
Delay or neglect in the diagnosis or treatment of appendicitis can be measured either directly or indirectly by surrogate markers such as perforation rates and mass formation. But there is no universally agreed definition as to what can be termed as ‘neglected appendicitis’. Goyal et al. defined it as missed diagnosis when the patient had visited an ED at least once within the preceding 7 days of correct diagnosis [13]. Some authors fixed the cutoff point as 48 hours [14, 15]. Others disagreed with these high cutoff points as the incidence of perforation in children rose from 10% at 18 hours to 44% at 36 hours of symptom-onset [16]. The frequencies of Grade 1 to 4 appendicitis were 94%, 0%, 3%, and 3%, respectively if the total delay (symptom onset to operation) was within 12 hours; while the respective frequencies of disease severity changed to 60%, 7%, 27%, and 6% between 48 and 71 hours and to 54%, 7%, 26%, and 13% if the interval is longer than 71 hours [17].
Several studies and meta-analyses have shown that a brief in-hospital delay within 24 hours of admission is not associated with increased complication rates [18, 19, 20, 21]. Contrary to this other authors have noted complication rates increased from 6% with an in-hospital delay of 6 hours to 14% at 12–18 hours [22, 23]. It is also noted that pre-hospital delays are more detrimental than in-hospital delays, probably because of the protection offered by antibiotics in admitted patients [5].
Considering the foregoing facts, inflamed appendix vermiformis can be defined as ‘neglected appendicitis’ if its diagnosis or treatment is delayed for more than 36 hours from the onset of symptoms [24, 25]. We do not find any practical advantage of further splitting the cut off points into pre-hospital delay of 24 hours and in-hospital delay of 12 hours.
It is important not to confuse ‘criminal neglect’ with ‘clinical neglect’; the former is contrived, ill-intended and punishable by court of law while the later is mostly inadvertent, circumstantial or even deliberately professed for the well being of patients. ‘Clinical neglects’ are often due to inherent limitations of medical science, complexities of human pathophysiology or socioeconomic factors. It is important that clinical neglects be reduced by training, education and welfare measures rather than by punishments, lest it will backfire on the society.
3. Factors of delay
Delay may occur in the diagnosis or treatment of acutely inflamed appendix and the former may occur in pre-hospital setting or after admission to a hospital. Factors of delay may be patient-related or physician-related. Some of them are modifiable while others are non-modifiable.
3.1 Age of patients
Extremes of age are more liable for pre-hospital diagnostic delays [9, 26, 27]. Dependency on caretakers for hospital visits, inability to accurately express symptoms (either because of dementia or immaturity), absence of economic freedom and altered pain perception are common in both infants and elderly [26]. The younger the child, the more difficult is an accurate diagnosis. None of the neonatal appendicitis was diagnosed preoperatively and a mean delay of 8 days (5–11 days) is not unusual in this age group [28]. Age less than 30 years [5] and that above 55 years [29] are found to be associated with significant pre-hospital delays. A mean delay of 3 days (2–5 days) is usual in elderly patients [30].
3.2 Sex of patients
Several authors [5, 29] found male gender at a higher risk of pre-hospital delay while others [14, 31] found females more vulnerable. Gender bias and discrimination of girl children is not unknown in many underdeveloped countries. Besides this, delay in adult females is also partly due to non-classical symptoms and closely mimicking differential diagnosis such as ectopic pregnancy [31].
3.3 Rural and remote population
Access to health care facility is often a challenge for rural population [5, 32, 33] and remote inhabitants such as those of Himalayan high altitude [34]. In Australia, rural families had to travel a mean distance of 50 km for consulting a physician and it resulted in a mean delay of 42 hours [35]. Transportation delay was as much as 3 to 7 days in rural Africa. The mean ambulance transport time from district hospital to referral hospital was as much as 4.9 hours [33]. Rural people treated at an urban facility have more frequent perforations than rural people treated at local rural hospitals. This is suggestive of the detrimental effect of delayed transportation [36]. Even in countries with air ambulance service, significant delay is attributed to physician disagreement on transfers and data connectivity [37].
Rural people are also more likely to have superstitious beliefs which prevent them from seeking timely medical help [38]. Some of the primitive tribes exhibit trust deficit with urban hospitals and modern medicine. For example, the cultural safety and hostility of Maori tribe of New Zealand and Jarawa tribe of Andaman Islands remains a problem [35]. It was also suggested that increased complication in rural children may be due to more severity of the disease per se rather than due to transportation delay [39]. There are some data showing that the urban–rural gap is now started narrowing in some countries [40].
3.4 Race and ethnicity
Perforated appendicitis is more common in Black children due to delayed treatment as compared to that of Hispanics [41]. Black children with appendicitis are subjected to less frequent imaging than non-Hispanic Whites. This may be due to racial discrimination or due to insurance status [13]. Maori children of Australia had more perforation (28.9%) than White children (19%) [39]. Some authors deny the existence of any racial difference or discrimination [42].
3.5 Duration of symptoms
Those with shorter duration of symptoms at initial visit are more likely to be misdiagnosed. About 69% of those who visited ED within 24 hours of symptom onset were misdiagnosed [43]. This paradox can be explained by the atypical nature of evolving symptoms that overlap with many other non-surgical disorders. In delayed cases, diagnosis becomes self-evident by the established signs of complications such as peritonitis [22].
3.6 Weak-end and wee-hour presentations
Patients who present in midnight and wee-hours are often misdiagnosed [43]. They typically undergo less investigations and stay for shorter period in ED. Non-availability of the services of senior consultants, and physical exhaustion of ED personnel could contribute to diagnostic delays in wee-hours. Circadian variation in surgeons’ ability to diagnose appendicitis was, however, denied by Danish researchers [44]. Children who presented on Mondays had more risk of perforation [45]. Interestingly, the odds of perforation were 30% higher in those who presented in working hours (9 am – 3 pm) than in those who presented in wee hours [46]. These paradoxes can be explained by the challenges of transportation in weekends and wee hours.
3.7 Self medications
As much as 23% of pre-hospital delay was due to self medications and home remedies tried by patients [47]. The risk is especially more if father is the caregiver [48]. Over the counter sale of antibiotics, which is common in many southeast Asian and African countries, partially mask the symptoms thereby contributes to diagnostic delays [48]. Interestingly, in Nigeria, antibiotic self-medication did not adversely affect outcome despite causing significant pre-hospital delay [49]. Beneficial effect of antibiotics in controlling infection appears to compensate the detrimental effect of delayed hospital admission. Early empirical usage of non-steroidal analgesics but not opioid analgesics was found to cause delay in seeking medical help [50].
3.8 Season
In a rural population, complicated appendicitis was more common in winter (75%) than in other seasons (33%) [51]. Interestingly, appendicitis is generally accepted to be more common in summers than in winters. For every increase of 5.56°C of environmental temperature, the incidence of appendicitis increases by 1.3% [52]. Therefore the observed seasonal difference in complications may be attributed to the challenges of transportation in winter.
3.9 Educational and socioeconomic status
Pre-hospital delay is significantly reduced in educated population. Physicians and other medical workers develop perforation less often than general population [53]. It was suggested that insurance status rather than actual socioeconomic status of patients influences the diagnostic and therapeutic delay. For obvious reasons, uninsured patients often present late and they undergo less investigation than insured citizens [53, 54]. However, data from one American center denied any correlation between the frequency of appendicular perforation and educational level, income or insurance status [42].
3.10 Pandemics
Lockdown of covid-19 pandemic posed enormous challenges in accessing health care services and caused inordinate delay in treatment [55, 56, 57]. Frequency of perforation (38 vs. 21%), gangrene (23 vs. 16%) and peri-appendicular abscess (5 vs. 1%) were higher during pandemic time than during the preceding year [55]. Number of consultations for acute appendicitis decreased by 20% as compared to pre-pandemic times [56]. Pre-hospital delay as long as 2 days [57] was suggesting either transportation difficulties or reluctance of patients in attending hospitals. On the other hand, co-existence of Covid-19 caused severe conflicts in therapeutic decision making and thereby contributed to in-hospital delay. Exhaustion of hospital resources, risk of spreading covid-19 infection to the surgical team, unknown implications of operating upon a Covid-19 patient and financial hardship of lockdown frequently resulted in postponement of appendicectomy. Most often patients were managed non-operatively with antibiotics although it is clearly undesirable at other times [56]. Interestingly, a children’s hospital in Spain did not find any significant difference in pre-hospital symptom duration, laboratory investigation, hospital stay, intensive care admissions or diagnostic errors during the pandemic [58].
3.11 Coexisting diseases
Not infrequently presence of coexisting diseases causes delay in the management of appendicitis by masking or mimicking the classical symptoms, by posing conflicts of priority in intervention, by interfering with imaging and laboratory diagnosis and by preventing access to health care services. For example, inability to express symptoms, altered pain perception and dependency on care takers to reach hospital are common in neurological disorders such as dementia, schizophrenia, mental retardation, deaf mutism and cerebral palsy [59]. However, a Taiwanese study refuted any association between mental disorders and therapeutic delays in acute appendicitis [60]. Altered perception of pain due to visceral neuropathy, associated nephropathy, atypical symptoms that overlap with ketoacidosis are known to cause significant diagnostic delay in diabetics [61]. Obesity is known to interfere with clinical diagnosis and surgical approach of acute appendicitis [62].
Aortic aneurysm, myocardial infarction, Henoch-Schonlein Purpura, myelodysplasia, leukemia, lymphoma, carcinoid tumors, cecal carcinoma, Kawasaki disease, gastroenteritis, typhoid fever and measles are frequently know to coexist with appendicitis and cause serious therapeutic dilemma. Adverse effects of drugs used to treat coexisting diseases such as chemotherapy of cancers, hypertension and end-stage renal disease pose serious therapeutic dilemma thereby cause considerable delay in doing appendicectomy [63]. Appendicitis secondary to blunt injury of abdomen (post-traumatic appendicitis) is invariably mistaken for hemoperitoneum; however, fortunately therapeutic interventions are not delayed in such patients [64].
3.12 Mimicking diseases
Although most of the diagnostic delays occur at the level of primary care physicians, specialist consultants at tertiary care centers are not immune to this. Physician related diagnostic delays are often due to the dilemma caused by overlapping symptoms of acute appendicitis with that of other abdominal emergencies. The list of differential diagnosis of acute appendicitis is very long including several infectious diseases, genitourinary pathologies, metabolic disorders and malignancies [65]. Recently, Covid-19 related MISC (Multisystem Inflammatory Syndrome in Children) was reported to mimic appendicitis [66]. Better imaging and liberal use of laboratory investigations may avoid diagnostic errors and in-hospital delays.
3.13 Pregnancy
Typical clinical presentation of appendicitis is seen in only 50 to 60% of pregnant women [67]. Approximately 50% of appendicitis occur in the second trimester [67]. The frequency of perforated appendicitis in the first, second and third trimester are 8.7%, 12.5%, and 26.1% respectively [67]. The symptoms of early appendicitis, such as nausea and vomiting, are also seen in the morning sickness of pregnancy. The normal febrile response to appendicular infection may be blunted in pregnancy [68]. Classical tenderness at McBurney’s point may be absent in pregnancy as the gravid uterus causes cephalad or posterior displacement of the appendix. Lower quadrant pain of the second trimester produced by traction on the suspensory ligaments of the uterus, a phenomenon known as round ligament pain, may be confused with appendicular pain [69]. Choice of imaging is also restricted in pregnancy as CT scan cannot be used due to concerns of radiation exposure. Gravid uterus overlying the appendix will also interfere with adequate sonographic imaging. Finally, laboratory tests such as leucocytosis that are typical of appendicitis are commonly seen during normal pregnancy. However, pain in the right lower quadrant of the abdomen remains the cardinal feature of appendicitis in pregnancy. Ectopic pregnancy or uterine contractions many also mimic or coexist with appendicular pain. Fetal loss occurs in 3–5% of uncomplicated appendicitis which increases to 20% in perforated cases. Laparotomy especially in first and third trimester carries the risk of abortion or premature delivery [70]. Because of these reasons, a subconscious reluctance is often noted among surgeons in diagnosing acute appendicitis and operating upon pregnant women.
3.14 Physician of first contact
Patients consulting an appropriate specialist during first visit are less likely to be misdiagnosed than those who consult general physicians or family practitioners [71, 72]. The later more often tend to diagnose medical disorders and treat conservatively than surgeons who swiftly decide on surgical operation [73]. Physicians who omit imaging or WBC counts are more likely to miss the diagnosis [4]. When the initial physician missed the diagnosis, the perforation rate increased from 20 to 31% [4].
3.15 Choice of initial imaging
Patients who received only an abdominal x-ray rather than a ultrasonography or a CT scan are more likely to be misdiagnosed [74]. Presence of local ileus often precludes penetration of ultrasound waves leading to non-visualization of the appendix. In doubtful cases, laparoscopy may be used both as a diagnostic as well as a therapeutic tool. Diagnostic accuracy of laparoscopy approaches 98% and it picks up even cases that are missed in CT scan or sonography [75]. MRI is an alternative for accurate diagnosis but it requires interpretation by an imaging expert [76].
3.16 Anatomical location of appendix
Clinical features of acute appendicitis are partly determined by the anatomical position of the organ. Among orthotopic appendices, retrocecal position notoriously masks local tenderness and presents with backache [77]. Pre- or post- ileal appendices may be mistaken for gastroenteritis and pelvic appendix for urinary tract infection [78].
Correct diagnosis is invariably missed on first ED visit if the appendix is ectopically located. Subhepatic appendicitis is often mistaken for cholecystitis [79]. Only 50% of patients had a correct preoperative diagnosis when the appendix was located on the left-iliac fossa or epigastrium as it is in situs inversus or midgut malrotation [80]. Intrathoracic appendix associated with diaphragmatic hernia may mimic acute chest pain [81]. Herniated appendix into the scrotum (Amyand hernia) may be mistaken for epididymo-orchitis or testicular torsion [82]. In all these cases diagnosis is seldom made on first ED visit.
3.17 In-hospital therapeutic delays
Despite admission to hospital, surgical intervention is often delayed for several reasons which include diagnostic dilemma, waiting for emergency operation slot and interruption of therapeutic plans by co-existing diseases [83]. Fortunately in-hospital delays as long as 24 hours are found to be safer than pre-hospital delays [84]. This is because of the administration of intravenous antibiotics under watchful eyes [85]. If an appendectomy is done on the day of admission, the perforation rate was 28.8% while it increased to 33.3% on day 2 and 78.8% by day 8 [12].
Inordinate delay in the diagnosis and intervention may also happen in postoperative appendicitis that develops in 0.1% of patients undergoing major surgery [86]. Diagnosis will be extremely difficult if the original surgery was a laparotomy. It may occur 5–31 days after the primary operation and its diagnosis is delayed 12 hours to 8 days [86]. These patients required hospitalization for as long as 80 days.
4. Prevention of diagnostic delays
Diagnostic delays are significantly reduced when the primary physician performs the triad of leukocyte count, C-reactive protein and ultrasonography [87]. Several scoring systems have also been proposed to minimize diagnostic ambiguities. Alvarado score in adults and Samuel score in children are very popular [88]. There are also computer driven algorithms such as Eskelinen Scoring and online Pediatric Appendicitis Risk Calculator (PARC) [Kharbanda - https://www.mdcalc.com]. Role of artificial intelligence in improving the diagnostic accuracy is currently under validation [89, 90]. Resource allocation to improve ambulance services and hospital infrastructure may avoid in-hospital delays but their implementation will continue to be a challenge especially in underdeveloped countries.
5. Consequences of neglected appendicitis
5.1 Perforation
With delay in diagnosis or treatment, appendicular inflammation progresses to cause perforation [91]. Pathogenic mechanisms of perforation are diverse including inflammatory suppuration of the appendicular wall, gangrene secondary to thrombosis of the appendicular artery and rupture due to built-up intraluminal pressure of fermented gases or pent-up secretions [92]. Perforation causes spillage of fecal matter and infected luminal contents in to the peritoneal cavity. Consequences of the spillage depend up on the pattern of perforation: (1) In

Figure 2.
Fecolith (white arrow) causing necrosis and perforation (black arrow) of the tip of inflamed appendix (the appendicular blow-out).
5.2 General peritonitis
General peritonitis occurs mostly as a complication of appendicular perforation (Figure 3). But it can also occur in simple uncomplicated appendicitis. Clinical features of general peritonitis are unmistakable [95]. Extensive diagnostic work-up is irrelevant at this stage. Emergency laparotomy not only provides diagnostic solution but also the life saving therapy.

Figure 3.
Perforated appendicitis with general peritonitis.
5.3 Prolonged ileus
Prolonged ileus as a sequela of appendicular peritonitis is seen in 1–5% of patients [100]. Ileus may be due to bacterial toxins, electrolyte imbalance (hypokalemia), inflammatory edema or adverse effects of medications. More importantly, postoperative mechanical obstruction should not be mistaken for paralytic ileus. Consequences of such misdiagnosis are devastating as paralytic ileus is treated expectantly while mechanical obstruction necessitates prompt re-laparotomy. The duration of ileus is proportional to the duration, severity and extent of peritonitis. Some amount of ileus is common in the first 72 hours of appendicectomy. But if it prolongs beyond 72 hours it should be considered pathological [101]. The senior author has seen ileus persisting for 15 days. Prokinetic agents such as metoclopromide may be useful in stimulating the paralyzed bowel.
5.4 Appendicular mass (phlegmon)
Appendicular mass is composed of ileum, cecum, mesentery, fallopian tube and omentum that are adherent to the inflamed appendix. It may occur with or without perforation. Appendicular mass is usually formed after 72 hours from the onset of symptoms [102]. Attempted appendicectomy at this stage is more likely to cause collateral damage to the cecum and the adherent bowel which are rendered friable by inflammation (Figure 4). Therefore, general recommendation is to treat appendicular mass conservatively with Ochsner Sherren regimen [103, 104]. Presence of fecolith, leukocyte count >15,000/mm [3], bandemia, CT scan showing extension of mass beyond the limits of the right lower quadrant are factors that contraindicate non-operative management of appendicular mass.

Figure 4.
Fecal fistula following attempted appendicectomy in a boy with appendicular mass.
Further fate of the mass is determined by the virulence of infection, presence of perforation and success of antimicrobial treatment. If medical treatment is successful, the appendicular mass will resolve. On the other hand, progression of suppurative infection transforms the mass into an abscess.
5.5 Appendicular abscess
Appendicular abscess is collection of pus within appendicular mass due to ongoing infection [105]. It may occur with or without perforation. It may be intraperitoneal, retroperitoneal or pelvic depending on the anatomical position of the appendix. In addition to administration of broad spectrum antibiotics, the pus has to be surgically drained. Temptation to do appendicectomy at the time of abscess drainage is better desisted as adherent bowel loops will be friable leading to operative injury [106, 107]. Even intraperitoneal abscesses can be drained by extraperitoneal approach as the adhesions effectively seals the abscess cavity from rest of the peritoneal cavity. Pelvic abscess can be drained per rectally.
If proper treatment is neglected, appendicular abscess may rupture into the peritoneal cavity causing general peritonitis, into hollow viscera causing internal fistula or may rupture externally causing fecal fistula. Spread of infection may also result in intra-peritoneal or metastatic abscesses, meningitis, endocarditis, myocarditis and Systemic Inflammation Response Syndrome (SIRS).
5.6 Metastatic abscess
Direct or hematogenous spread of infection from the appendix is known to cause metastatic abscesses in adjacent or distant organs. Anatomical drainage of the appendicular vein into the portal vein facilitates lodging of septic emboli in the liver parenchyma causing liver abscess [108]. Direct fistulation of appendix into the liver has also been reported [109]. Seepage of infected fluid through the patent processes vaginalis or femoral canal can result in scrotal or right thigh abscess respectively [110, 111]. Rupture of liver abscess into pleural space or hematogenous spread of infection may cause empyema or lung abscess [112]. Metastatic abscesses of CNS [113], spleen [114], kidneys [115] are also known to complicate neglected appendicitis.
5.7 Fecal fistula
Apart from iatrogenic cecal injuries during appendicectomy, spontaneous rupture or surgical drainage of appendicular abscess may also result in fecal fistula. Such fistulae may be internal or external. Appendico-vesical fistula (n = 120) is the most common type of internal fistula complicating appendicitis [116]; followed by appendico-rectal [117], appendico-ileal [116], appendico-colic [118] fistulae. External appendico-cutaneous fistulae may occur in loin [119], thigh, groin [120] or umbilicus [121]. Usually, fecal matter and pus from suppurated appendicitis dissect their way externally and present as simple soft tissue abscess. Upon surgical drainage of such unsuspected abscess, fecal fistula will ensue. Multiple aerobes and anaerobes of the fecal fistula may cause extensive necrotizing fasciitis - also known as Meleney’s ulcer [122]. Co-existing actinomycosis, Crohn’s disease, luminal obstruction by tumors or fecolith and tuberculous enteritis favors the development of fecal fistula complicating appendicitis [123].
Fistulogram or CT scan is essential for diagnosing fecal fistula (Figure 5). Prompt appendicectomy, with or without cecal resection, is necessary to cure the fistula. However, appendico-colic fistulae may be managed conservatively and they require surgical intervention only if symptomatic.

Figure 5.
Fistulogram delineating appendico-cutaneous fistula.
5.8 Intestinal adhesive obstruction
Small bowel adhesion leading to intestinal obstruction is not an uncommon sequela of appendicitis if overall therapeutic delay is more than 36 hours (Figure 6) [124]. Adhesions of inflamed appendix may also form a knot around the intestinal loops (appendicular knotting) causing strangulation and gangrene of small bowel [125].

Figure 6.
Frozen peritoneum following perforated appendicitis. It is an extensive form of bowel adhesions and peritoneal plastering.
5.9 Stump appendicitis
Inadvertent failure to do complete excision leaves behind a stump of the appendix that may subsequently get inflamed. It occurs in 0.25% of appendicectomy and it is more common with laparoscopic excision [126]. Factors responsible for incomplete appendicectomy are lack of tactile feedback during laparoscopy, extensive plastering of appendix to cecal wall thereby obscuring its anatomical limits and inexperience on the part of surgeon. History of prior appendicectomy often precludes stump appendicitis from being considered as a differential diagnosis of recurrent abdominal pain. Although excision of the stump was sufficient in 94%, about 6% patients required extended resection of cecum to address the problem [126].
5.10 Pyelephlebitis
Septic thrombophlebitis of the portal vein is one of the most serious complications of neglected appendicitis. Its incidence is 0.05% in simple appendicitis and it dramatically increases to 3% in delayed cases [127]. This rare complication should be suspected in appendicitis if the patient presents with a high fever and mild jaundice. Doppler or CT scan will demonstrate thrombus in the portal vein. Despite aggressive treatment, hepatic abscesses (50%) and mortality (30 to 50%) are not uncommon. Survivors of acute pyelephlebitis may develop portal hypertension, cavernous transformation of the portal vein and esophago-gastric varices.
6. Choice of imaging in neglected appendicitis
Ultrasound is the diagnostic investigation of choice in early as well as neglected appendicitis [128]. It is simple, easily available, cost-effective and is without radiation hazard. However, overlying bowel gas may interfere with the transmission of ultrasound waves and preclude sonographic visualization of the organ in case of appendicular mass or retrocecal appendicits. In such cases, contrast enhanced CT (CECT) scan will be useful [129]. Presence of peri-appendicular fluid or pus collection, extraluminal or intramural gas, appendicular wall enhancement defect, peri-appendiceal fat stranding, ascites, local ileus and free floating or luminal fecolith are the features that differentiate neglected (complicated) appendicitis from simple appendicitis (Figure 7) [129]. MRI does not appear to offer any added advantage over CECT in neglected appendicitis. It is often suggested that conditional CT scan combined with ultrasonography is beneficial (Table 1).

Figure 7.
Contrast enhanced CT scan showing appendicular mass (arrow).
| Imaging modality | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| Ultrasound (USG) | 32–86% | 60–93% | 42–60% | 80–93% |
| CT scan (CECT) | 28–95% | 71–100% | 74% | 93% |
| MRI | 37–75% | 77–92% | 58% | 86% |
| USG + CECT | 30–67% | 85–97% | 70% | 84% |
Table 1.
* Neglected appendicitis includes complicated pathologies such as perforation, phelgmon and abscess. These figures are compiled from the meta-analysis of Bom et al. [128]. CECT – Contrast enhanced computed tomography, MRI-Magnetic resonance imaging.
7. Management of neglected appendicitis
7.1 Ochsner Sherren (non-operative) regimen
Although Claudius Amyand performed the first successful appendicectomy in 1735 CE, its popularity catapulted only after 1902 CE when King Edward VII underwent dramatic surgical drainage of appendicular abscess just before his coronation. It had even become fashionable among English nobility to request for prophylactic appendicectomy. Therefore, no one thought of non-surgical management of inflamed appendix. Ironically in the same year (1902 CE) Albert Ochsner of Chicago first suggested that conservative management followed by interval appendicectomy is better than immediate operation of appendicular mass. Later in 1905 James Sherren of London modified and popularized Ochsner’s treatment which came to be known as ‘Ochsner Sherren’ regimen.
Ochsner Sherren regimen [130] consists of high Fowler’s (propped-up) position, strict monitoring of pulse and vomiting, only sips of plain water orally for 5 days followed by gradual restarting of semisolid diet, absolute ban on enema and avoiding any drugs especially those that could mask pain, vomiting or diarrhea. The size of the appendicular mass is marked on the skin and its size-reduction is monitored. Patients with hyperesthesia, age less than 5 years, uncertainty of diagnosis, general peritonitis and those who had received purgatives are exempted from this treatment. The regimen is abandoned in favor of laparotomy if pulse rate increases, if pain persists, if the lump is not getting smaller after 5 days of treatment, if the lump becomes visible when viewed tangentially, if the mass becomes fluctuant, if the temperature is swinging or if a pelvic abscess is palpated. Hamilton Bailey typically described the indication of Ochsner-Sherren regimen as “appendicitis that is too late for the early operation and too early for the late operation” [130]. In Bailey’s series, out of the 73 patients treated non-operatively only one died. This impressively low mortality of 1.4% was considered a wonder in pre-antibiotic era [130]. However, keeping up with the popular trend, an interval appendicectomy was advised after 8 weeks.
Despite the discovery of antibiotics in 1920s and their wide availability in 1940s, Ochsner-Sherren regimen remained popular until 1980s just because it was endorsed by none other than the venerable Hamilton Bailey and McNeil Love in their universally popular textbook, ‘Short Practice of Surgery’. Bailey and Love’s advice was backed by lot of wisdom. They advised routine appendicectomy if the duration of symptoms was less than 48 hours beyond which Ochsner Sherren regimen was recommended. This is because attempted separation of appendix from adhesions would result in fecal fistula and death due to operative injury to friable bowel loops.
Appropriateness of Ochsner Sherren regimen was reexamined in the era of newer antibiotics and advanced surgical techniques. Results are conflicting: some studies [131, 132, 133] claim cost effectiveness and low complication rates with non-operative treatment while others [134, 135] found a clear advantage of immediate appendicectomy even in delayed cases. A meta-analysis noted increased complication with surgical management and less efficacy with exclusive antibiotic therapy [135]. A Turkish study found the length of hospital stay was longer in conservative group while the cost of care was increased in operated patients. However, morbidity and mortality were similar in both groups [131]. Taiwanese surgeons found shorter duration of fever (2.7 vs. 8.0 days), delayed oral feeds (4.4 vs. 1.8 days), higher complication rate (33 vs. 17%) among emergently operated children as compared to those who were treated conservatively [132].
Interest in conservative treatment is recently rekindled by Covid-19 pandemic. Due to concerns of covid-19 infection, appendicitis was mostly treated non-operatively during the pandemic [136, 137]. Interestingly, this time, the scope of non-operative treatment is extended even to early cases and is not restricted to just appendicular phelgmon as it was originally proposed by Ochsner and Sherren. It is even suggested that conservative management may become the choice of treatment of all acute appendicitis irrespective of diagnostic or therapeutic delays [138].
7.2 Interval appendicectomy
Following conservative treatment recurrence of appendicitis was noted in 24% of children at a mean follow up of 16 months [139] and in 19% of adults at 33 months [140]. This prompted the recommendation of elective interval appendicectomy. However its indispensability was recently questioned [102, 141, 142, 143]. A meta-analysis found increased complications with early appendicectomy while interval appendicectomy incurred more cost [105].
The only feared complication of acute appendicitis is its rupture and life-threatening fecal peritonitis. However, there are some data to suggest that successive episodes of recurrent appendicitis are progressively less severe with negligible risk of perforative peritonitis [144]. This is because the inflamed appendix heals by fibrosis and peri-appendiceal adhesions effectively contain any spillage. With each episode of inflammation the appendix gets progressively fibrosed and eventually becomes a fibrous cord. Therefore, it appears prudent to forego interval appendicectomy in favor of wait-and watch or repeated conservative management. However, we would recommend elective interval appendicectomy in selected cases such as remote rural population, more than 2 attacks that require hospitalization, children below 8 years and presence of serious co-existing diseases.
7.3 Mucosal appendicectomy
Deliberate excision of appendix by dissecting the appendicular mass, though not desirable, has to be occasionally performed for various reasons. Sometimes, even after surgical drainage of appendicular abscess, sepsis will not abate because of ongoing appendicular inflammation or leakage of fecal matter into the abscess cavity. In such cases appendicectomy is inevitably essential to control sepsis. However dense adhesions make surgical approach hazardous. Attempted separation of appendix from adjacent structures is sure to invite operative injury and fecal fistula. To avoid this trouble, Raveenthiran has described a new technique called MUCOSAL appendicectomy (Figure 8) [145].
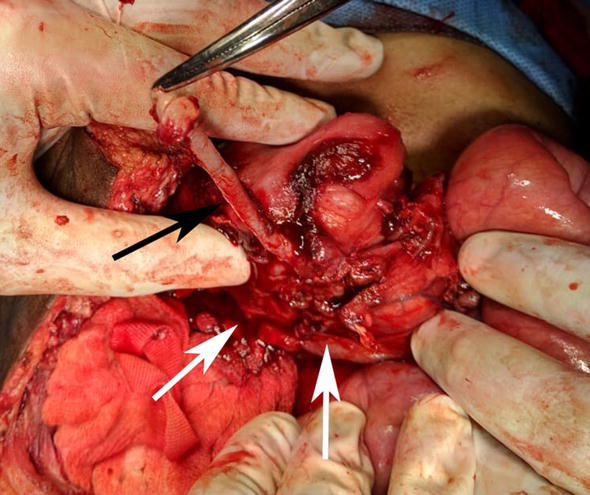
Figure 8.
MUCOSAL appendicectomy. Mucosal tube of appendix (black arrow) is being dissected off its muscular cuff (white arrows).
The concept was first hinted in 2006 by Rangarajan et al. and in 2020 Raveenthiran described the technique in great detail and named it as ‘MUCOSAL appendicectomy’. The nomenclature is an acronym of ‘mucosa coring salvage appendicectomy’. This technique is also variously known as ‘subserosal’, ‘submucosal’ or ‘trans-mesoappendicular’ appendicectomy [145]. In this technique, the appendix is exposed by gentle finger dissection of adhesions (Figure 9). However, no attempt is made to isolate it from cecum or other adherent structures. A longitudinal sero-muscular incision is made and the mucosal tube is gently dissected off the muscular cuff similar to the dissections of Soave’s endorectal pull-through operation or Lilly’s operation for choledochal cyst. The dissected mucosal tube is ligated at its base and the redundant portion is excised. The seromuscluar cuff is left undisturbed. As appendicitis is essentially a disease of mucosa, removal of mucosal layer rather than the whole appendix is sufficient to prevent further attacks. Contrary to expectations, dissections in submucosal plane do not cause significant bleeding, as the tiny blood vessels are occluded either by thrombosis or by inflammatory edema. Therefore, it is safe to perform MUCOSAL appendicectomy even in the presence of disseminated intravascular coagulation syndrome.

Figure 9.
Diagrammatic illustration of the principles of MUCOSAL appendicectomy. (A - Seromuscular incision over the appendix adherent to cecum; B - Dissection of mucosal tube; C - Completion of MUCOSAL appendicectomy leaving behind the seromuscular cuff).
7.4 Role of laparoscopy
The role of minimally invasive surgery (laparoscopy) in neglected appendicitis is slowly evolving [146, 147]. Long incisions of open surgery, that are required for better surgical access of adherent appendix, can be negated in laparoscopy. Thus, the risks of wound site infection, incisional hernia, hospital stay are low with laparoscopic approach. On the other hand, pneumoperitoneum of laparoscopy is thought to cause widespread dissemination of infection which would have otherwise remained limited to the right lower quadrant. For this reason, intra-abdominal abscesses are more common with laparoscopic appendicectomies [148].
8. Outcome of neglect
Death due to appendicitis is now rare and it is 0.07 to 0.1% [149] or 4 per million-population [150]. However, with a pre-hospital delay of 1 week or more, mortality increases to 5.6% [151]. Death rate reaches as high as 20% when delay is combined with co-morbidities such as cardiovascular diseases [149]. Maternal mortality increases by fivefold and fetal loss by twofold when delay occurs in appendicitis of pregnancy [152]. Premature labor or abortion occurs in 15% of neglected appendicitis [152].
9. Medico-legal issues of neglect
Missed or delayed diagnosis of acute appendicitis is the second most common cause of legal litigations against emergency physicians [153]. Liberal use of multidetector computed tomography and magnetic resonance imaging does not appear to reduce the risk of misdiagnosis [154]. Therefore, meticulous history and physical examination remains indispensible even in this era of advanced technology. Nearly 50% of litigations are related to post-operative complications, 20% to misdiagnosis and 7% to intra-operation mishaps [155]. In litigious cases patient-related delay ranges from 12 hours to 45 days while physician-related delay ranges from 2 to 430 hours. Interestingly, physician-related delays are relatively safer and shorter than patient-related delays [155]. Therefore, in case of diagnostic uncertainty, it is better to admit the patient for in-hospital monitoring. With passage of time, diagnosis of many cases that were initially ambiguous will become self-evident by the evolution of classical features [155].
Despite prompt diagnosis and meticulous execution of therapeutic interventions morbidity and mortality of appendicitis cannot be completely annulled. Unexpected or unfortunate outcomes prompt aggrieved patients to legally challenge the quality of care. In such cases physician may take defense in obtaining informed consent, recording the diagnostic difficulties and therapeutic dilemmas and by doing necessary investigations. Defense may also be taken by emphasizing lack of motives and ill intensions.
References
- 1.
Galai T, Beloosesky OZ, Scolnik D, et al. Misdiagnosis of acute appendicitis in children attending the emergency department: The experience of a large, tertiary care pediatric hospital. European Journal of Pediatric Surgery. 2017; 27 :138-141 - 2.
Ferris M, Quan S, Kaplan BS, et al. The global incidence of appendicitis: A systematic review of population-based studies. Annals of Surgery. 2017; 266 :237-241 - 3.
Naiditch JA, Lautz TB, Daley S, et al. The implications of missed opportunities to diagnose appendicitis in children. Academic Emergency Medicine. 2013; 20 :592-596 - 4.
Graff L, Russell J, Seashore J, et al. False-negative and false-positive errors in abdominal pain evaluation: Failure to diagnose acute appendicitis and unnecessary surgery. Academic Emergency Medicine. 2000; 7 :1244-1255 - 5.
Alyhari Q , Ahmed F Sr, Nasreldin M, et al. Prehospital delay and its associated factors in Sudanese patients presenting with acute appendicitis at a teaching hospital. Cureus. 2022; 14 :e23036 - 6.
Flum DR, Morris A, Koepsell T, Dellinger EP. Has misdiagnosis of appendicitis decreased over time? A population-based analysis. JAMA. 2001; 286 :1748-1753 - 7.
Flum DR, McClure TD, Morris A, Koepsell T. Misdiagnosis of appendicitis and the use of diagnostic imaging. Journal of the American College of Surgeons. 2005; 201 :933-939 - 8.
Naoum JJ, Mileski WJ, Daller JA, et al. The use of abdominal computed tomography scan decreases the frequency of misdiagnosis in cases of suspected appendicitis. American Journal of Surgery. 2002; 184 :587-589; discussion 589-90. DOI: 10.1016/s0002-9610(02)01086-3 - 9.
Rothrock SG, Skeoch G, Rush JJ, Johnson NE. Clinical features of misdiagnosed appendicitis in children. Annals of Emergency Medicine. 1991; 20 :45-50 - 10.
Reynolds SL. Missed appendicitis in a pediatric emergency department. Pediatric Emergency Care. 1993; 9 :1-3 - 11.
Yang Y, Guo C, Gu Z, et al. The global burden of appendicitis in 204 countries and territories from 1990 to 2019. Clinical Epidemiology. 2022; 14 :1487-1499 - 12.
Papandria D, Goldstein SD, Rhee D, et al. Risk of perforation increases with delay in recognition and surgery for acute appendicitis. The Journal of Surgical Research. 2013; 184 :723-729 - 13.
Goyal MK et al. Racial and ethnic disparities in the delayed diagnosis of appendicitis among children. Academic Emergency Medicine. 2021; 28 :949-956 - 14.
Kambouri K, Aggelidou M, Deftereos S, et al. What are the risk factors responsible for the delay in diagnosis of acute appendicitis in children? Eleven-year research from a single institution. Folia Med (Plovdiv). 2019; 61 :389-396 - 15.
Nomura O, Ishiguro A, Maekawa T, et al. Antibiotic administration can be an independent risk factor for therapeutic delay of pediatric acute appendicitis. Pediatric Emergency Care. 2012; 28 :792-795 - 16.
Narsule CK, Kahle EJ, Kim DS, et al. Effect of delay in presentation on rate of perforation in children with appendicitis. The American Journal of Emergency Medicine. 2011; 29 :890-893 - 17.
Ditillo MF, Dziura JD, Rabinovici R. Is it safe to delay appendectomy in adults with acute appendicitis? Annals of Surgery. 2006; 244 :656-660 - 18.
Kim SH, Park SJ, Park YY, Choi SI. Delayed appendectomy is safe in patients with acute nonperforated appendicitis. International Surgery. 2015; 100 :1004-1010 - 19.
Abou-Nukta F, Bakhos C, Arroyo K, et al. Effects of delaying appendectomy for acute appendicitis for 12 to 24 hours. Archives of Surgery. 2006; 141 :504-507 - 20.
Yardeni D, Hirschl RB, Drongowski RA, et al. Delayed versus immediate surgery in acute appendicitis: Do we need to operate during the night? Journal of Pediatric Surgery. 2004; 39 :464-469 - 21.
Bhangu A, (United Kingdom National Surgical Research Collaborative). Safety of short, in-hospital delays before surgery for acute appendicitis: Multicentre cohort study, systematic review, and meta-analysis. Annals of Surgery. 2014; 259 :894-903 - 22.
Ashkenazi I, Zeina AR, Olsha O. In-hospital delay of surgery increases the rate of complicated appendicitis in patients presenting with short duration of symptoms: A retrospective cohort study. European Journal of Trauma and Emergency Surgery. 2022; 48 :3879-3886 - 23.
Kovler ML, Pedroso FE, Etchill EW, et al. Prolonged In-hospital time to appendectomy is associated with increased complicated appendicitis in children. Annals of Surgery. 2022; 275 :1200-1205 - 24.
Bickell NA, Aufses AH, Rojas M, Bodian C. How time affects the risk of rupture in appendicitis. Journal of the American College of Surgeons. 2006; 202 :401-406 - 25.
Eldar S, Nash E, Sabo E, et al. Delay of surgery in acute appendicitis. American Journal of Surgery. 1997; 173 :194-198 - 26.
Cibert-Goton V, Kung VWS, McGuire C, et al. Functional and anatomical deficits in visceral nociception with age: A mechanism of silent appendicitis in the elderly? Pain. 2020; 161 :773-786 - 27.
Omling E, Salö M, Saluja S, et al. Nationwide study of appendicitis in children. The British Journal of Surgery. 2019; 106 :1623-1631 - 28.
Raveenthiran V. Neonatal appendicitis (part 1): A review of 52 cases with abdominal manifestation. Journal of Neonatal Surgery. 2015; 4 (1):4 - 29.
Augustin T, Cagir B, Vandermeer TJ. Characteristics of perforated appendicitis: Effect of delay is confounded by age and gender. Journal of Gastrointestinal Surgery. 2011; 15 :1223-1231 - 30.
Lunca S, Bouras G, Romedea NS. Acute appendicitis in the elderly patient: Diagnostic problems, prognostic factors and outcomes. Romanian Journal of Gastroenterology. 2004; 13 :299-303 - 31.
McGann Donlan S, Mycyk MB. Is female sex associated with ED delays to diagnosis of appendicitis in the computed tomography era? The American Journal of Emergency Medicine. 2009; 27 :856-858 - 32.
McCartan D, Fleming FJ, Grace PA. Perforated appendicitis: Does rural residency really explain the delay? Annals of Surgery. 2014; 259 :e58 - 33.
Kong VY, Aldous C, Clarke DL. Understanding the reasons for delay to definitive surgical care of patients with acute appendicitis in rural South Africa. South African Journal of Surgery. 2014; 52 :2-5 - 34.
Pande T, Mohanty Z, Nair A, et al. Seasonal variation of acute appendicitis: An armed forces experience of high altitude. Medical Journal, Armed Forces India. 2021; 77 :479-484 - 35.
Elliott BM, Bissett IP, Harmston C. Prehospital barriers for rural New Zealand parents in paediatric appendicitis: A qualitative analysis. ANZ Journal of Surgery. 2021; 91 :2130-2138 - 36.
Paquette IM, Zuckerman R, Finlayson SR. Perforated appendicitis among rural and urban patients: Implications of access to care. Annals of Surgery. 2011; 253 :534-538 - 37.
Edwards KH, Franklin RC, Edwards MT, Stewart RA. Requesting air ambulance transport of patients with suspected appendicitis: The decision-making process through the eyes of the rural clinician. The Australian Journal of Rural Health. 2022. DOI: 10.1111/ajr.12956 - 38.
Bagcchi S. Villagers in rural India stage anti-superstition march after boy dies from untreated appendicitis. BMJ. 2014; 348 :g1281 - 39.
Elliott BM, Witcomb Cahill H, Harmston C. Paediatric appendicitis: Increased disease severity and complication rates in rural children. ANZ Journal of Surgery. 2019; 89 :1126-1132 - 40.
Huang N, Yip W, Chang HJ, Chou YJ. Trends in rural and urban differentials in incidence rates for ruptured appendicitis under the National Health Insurance in Taiwan. Public Health. 2006; 120 :1055-1063 - 41.
Ladd MR, Pajewski NM, Becher RD, et al. Delays in treatment of pediatric appendicitis: A more accurate variable for measuring pediatric healthcare inequalities? The American Surgeon. 2013; 79 :875-881 - 42.
Nwomeh BC, Chisolm DJ, Caniano DA, Kelleher KJ. Racial and socioeconomic disparity in perforated appendicitis among children: Where is the problem? Pediatrics. 2006; 117 :870-875 - 43.
Chang YJ, Chao HC, Kong MS, et al. Misdiagnosed acute appendicitis in children in the emergency department. Chang Gung Medical Journal. 2010; 33 :551-557 - 44.
Jørgensen AB, Amirian I, Watt SK, et al. No circadian variation in Surgeons' ability to diagnose acute appendicitis. Journal of Surgical Education. 2016; 73 :275-280 - 45.
Deng Y, Chang DC, Zhang Y, et al. Seasonal and day of the week variations of perforated appendicitis in US children. Pediatric Surgery International. 2010; 26 :691-696 - 46.
Drake FT et al. Time-of-day and appendicitis: Impact on management and outcomes. Surgery. 2017; 161 :405-414 - 47.
Asad S, Ahmed A, Ahmad S, et al. Causes of delayed presentation of acute appendicitis and its impact on morbidity and mortality. Journal of Ayub Medical College, Abbottabad. 2015; 27 :620-623 - 48.
Castro P, Rincón J, Sánchez C, et al. Presurgical time and associated factors as predictors of acute perforated appendicitis: A prospective cohort study in a teaching pediatric hospital in Colombia. BMC Pediatrics. 2022; 22 :49 - 49.
Archibong AE, Ekanem I, Jibrin P. Appendicitis in south eastern Nigerian children. The Central African Journal of Medicine. 1995; 41 :94-97 - 50.
Frei SP, Bond WF, Bazuro RK, et al. Is early analgesia associated with delayed treatment of appendicitis? The American Journal of Emergency Medicine. 2008; 26 :176-180 - 51.
Özkurt E. Factors affecting patient outcomes in acute appendicitis in rural areas: An observational cohort study. World Journal of Surgery. 2021; 45 :2337-2346 - 52.
Simmering JE, Polgreen LA, Talan DA, et al. Association of Appendicitis Incidence with Warmer Weather Independent of season. JAMA Network Open. 2022; 5 :e2234269 - 53.
Deng CY, Huang N, Chou YJ, et al. Comparison of perforation risk among physicians, other medical professionals and general adults with acute appendicitis in Taiwan. The British Journal of Surgery. 2006; 93 :1297-1302 - 54.
Putnam LR, Tsao K, Nguyen HT, et al. The impact of socioeconomic status on Appendiceal perforation in pediatric appendicitis. The Journal of Pediatrics. 2016; 170 :156-60.e1 - 55.
Bickel A, Ganam S, Abu Shakra I, et al. Delayed diagnosis and subsequently increased severity of acute appendicitis (compatible with clinical-pathologic grounds) during the COVID-19 pandemic: An observational case-control study. BMC Gastroenterology. 2022; 22 :19 - 56.
Burgard M, Cherbanyk F, Nassiopoulos K, et al. An effect of the COVID-19 pandemic: Significantly more complicated appendicitis due to delayed presentation of patients! PLoS One. 2021; 16 :e0249171 - 57.
Gerall CD, DeFazio JR, Kahan AM, et al. Delayed presentation and sub-optimal outcomes of pediatric patients with acute appendicitis during the COVID-19 pandemic. Journal of Pediatric Surgery. 2021; 56 :905-910 - 58.
Gaitero Tristán J, Souto Romero H, et al. Acute appendicitis in children during the COVID-19 pandemic: Neither delayed diagnosis nor worse outcomes. Pediatric Emergency Care. 2021; 37 :185-190 - 59.
Lin HR, Wang HC, Wang JH, Lu HH. Increased risk of perforated appendicitis in patients with schizophrenia and dementia: A population-based case-control study. Medicine (Baltimore). 2020; 99 :e18919 - 60.
Hung SK, Kou HW, Wu KH, et al. Does medical disparity exist while treating severe mental illness patients with acute appendicitis in emergency departments? A real-world database study. BMC Psychiatry. 2022; 22 :488 - 61.
Tsai SH, Hsu CW, Chen SC, et al. Complicated acute appendicitis in diabetic patients. American Journal of Surgery. 2008; 196 :34-39 - 62.
Timmerman ME, Groen H, Heineman E, Broens PM. The influence of underweight and obesity on the diagnosis and treatment of appendicitis in children. International Journal of Colorectal Disease. 2016; 31 :1467-1473 - 63.
Chao PW, Ou SM, Chen YT, et al. Acute appendicitis in patients with end-stage renal disease. Journal of Gastrointestinal Surgery. 2012; 16 :1940-1946 - 64.
Cobb T. Appendicitis following blunt abdominal trauma. The American Journal of Emergency Medicine. 2017; 35 :1386.e5-1386.e6 - 65.
Dalpiaz A, Gandhi J, Smith NL, et al. Mimicry of appendicitis symptomatology in congenital anomalies and diseases of the genitourinary system and pregnancy. Current Urology. 2017; 9 :169-178 - 66.
Hwang M, Wilson K, Wendt L, et al. The great gut mimicker: A case report of MIS-C and appendicitis clinical presentation overlap in a teenage patient. BMC Pediatrics. 2021; 21 :258 - 67.
Ueberrueck T, Koch A, Meyer L, et al. Ninety-four appendectomies for suspected acute appendicitis during pregnancy. World Journal of Surgery. 2004; 28 :508-511 - 68.
Basaran A, Basaran M. Diagnosis of acute appendicitis during pregnancy: A systematic review. Obstetrical & Gynecological Survey. 2009; 64 :481-488 - 69.
Zachariah SK, Fenn M, Jacob K, et al. Management of acute abdomen in pregnancy: Current perspectives. International Journal of Women's Health. 2019; 11 :119-134 - 70.
Copson S, Nathan E. Surgery in pregnancy: Identifying factors contributing to variation and delay in diagnosis and management of appendicitis in pregnancy. The Australian & New Zealand Journal of Obstetrics & Gynaecology. 2021; 61 :500-504 - 71.
Bostanci SA, Şenel E. The importance of physician speciality on the diagnosis of acute appendicitis and its effect on morbidity in children. Journal of Paediatrics and Child Health. 2022; 58 :2003-2007 - 72.
Marzuillo P, Germani C, Krauss BS, Barbi E. Appendicitis in children less than five years old: A challenge for the general practitioner. World Journal of Clinical Pediatrics. 2015; 4 :19-24 - 73.
Bal A, Anil M, Nartürk M, et al. Importance of clinical decision making by experienced pediatric surgeons when children are suspected of having acute appendicitis: The reality in a high-volume pediatric emergency department. Pediatric Emergency Care. 2017; 33 :e38-e42 - 74.
Mahajan P, Basu T, Pai CW, et al. Factors associated with potentially missed diagnosis of appendicitis in the emergency department. JAMA Network Open. 2020; 3 :e200612 - 75.
Bachar I, Perry ZH, Dukhno L, et al. Diagnostic value of laparoscopy, abdominal computed tomography, and ultrasonography in acute appendicitis. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part A. 2013; 23 :982-989 - 76.
D'Souza N, Hicks G, Beable R, et al. Magnetic resonance imaging (MRI) for diagnosis of acute appendicitis. Cochrane Database of Systematic Reviews. 2021; 12 :CD012028 - 77.
Grunditz T, Rydén CI, Janzon L. Does the retrocecal position influence the course of acute appendicitis. Acta Chirurgica Scandinavica. 1983; 149 :707-710 - 78.
Castro BA, Novillo IC, Vázquez AG, et al. Impact of the appendiceal position on the diagnosis and treatment of pediatric appendicitis. Revista Paulista Pediatria. 2019; 37 :161-165 - 79.
Ball WR, Privitera A. Subhepatic appendicitis: a diagnostic dilemma. BML Case Reports. 2013; 2013 :bcr2013009454 - 80.
Akbulut S, Ulku A, Senol A, et al. Left-sided appendicitis: Review of 95 published cases and a case report. World Journal of Gastroenterology. 2010; 16 :5598-5602 - 81.
Parsons C, Naqvi S, Wheeler R. Intra-thoracic appendicitis in a child with Down's syndrome. Journal of Pediatric Surgery. 2013; 48 :E29-E31 - 82.
Raveenthiran V. Neonatal appendicitis (part 2): A review of 24 cases with inguinoscrotal manifestation. Journal of Neonatal Surgery. 2015; 4 (2):15 - 83.
van Dijk ST, van Dijk AH, Dijkgraaf MG, et al. Meta-analysis of in-hospital delay before surgery as a risk factor for complications in patients with acute appendicitis. The British Journal of Surgery. 2018; 105 :933-945 - 84.
Ingraham AM, Cohen ME, Bilimoria KY, et al. Effect of delay to operation on outcomes in adults with acute appendicitis. Archives of Surgery. 2010; 145 :886-892 - 85.
Yukumi S, Ishimaru K, Suzuki H, et al. Appropriate antibiotic selection during the in-hospital waiting period for surgery for appendicitis. Journal of Anus Rectum Colon. 2022; 6 :259-263 - 86.
Barr D, van Heerden JA, Mucha P. The diagnostic challenge of postoperative acute appendicitis. World Journal of Surgery. 1991; 15 :526-529 - 87.
Banerjee A, Ratan SK, Neogi S, et al. Role of ultrasonography and inflammatory markers in predicting complicated appendicitis. Journal of Indian Association of Pediatric Surgeons. 2022; 27 :448-454 - 88.
Sağ S, Basar D, Yurdadoğan F, et al. Comparison of appendicitis scoring Systems in Childhood Appendicitis. Turkish Achieves Pediatrics. 2022; 57 :532-537 - 89.
Ghareeb WM, Emile SH, Elshobaky A. Artificial intelligence compared to Alvarado scoring system alone or combined with ultrasound criteria in the diagnosis of acute appendicitis. Journal of Gastrointestinal Surgery. 2022; 26 :655-658 - 90.
Shikha A, Kasem A. The development and validation of artificial intelligence pediatric appendicitis decision-tree for children 0 to 12 years old. European Journal of Pediatric Surgery. 2022. DOI: 10.1055/a-1946-0157 - 91.
Holcomb GW, St Peter SD. Current management of complicated appendicitis in children. European Journal of Pediatric Surgery. 2012; 22 :207-212 - 92.
Andersson RE. The natural history and traditional management of appendicitis revisited: Spontaneous resolution and predominance of pre-hospital perforations imply that a correct diagnosis is more important than an early diagnosis. World Journal of Surgery. 2007; 31 :86-92 - 93.
Scher KS, Coil JA Jr. Appendicitis: Factors that influence the frequency of perforation. Southern Medical Journal. 1980; 73 :1561-1563 - 94.
Howell EC, Dubina ED, Lee SL. Perforation risk in pediatric appendicitis: Assessment and management. Pediatric Health Medical Therapy. 2018; 9 :135-145 - 95.
Bennion RS, Thompson JE, Baron EJ, Finegold SM. Gangrenous and perforated appendicitis with peritonitis: Treatment and bacteriology. Clinical Therapeutics. 1990; 12 (Suppl. C):31-44 - 96.
Obinwa O, Casidy M, Flynn J. The microbiology of bacterial peritonitis due to appendicitis in children. Irish Journal of Medical Science. 2014; 183 :585-591 - 97.
Rather SA, Bari SU, Malik AA, Khan A. Drainage vs no drainage in secondary peritonitis with sepsis following complicated appendicitis in adults in the modern era of antibiotics. World Journal Gastrointestinal Surgery. 2013; 5 :300-305 - 98.
Schietroma M, Piccione F, Carlei F, et al. Peritonitis from perforated appendicitis: Stress response after laparoscopic or open treatment. The American Surgeon. 2012; 78 :582-590 - 99.
Evbuomwan I, Onwanyin ON. Management of peritonitis in perforated appendicitis in children. East African Medical Journal. 1994; 71 :279-281 - 100.
Noble TB. Paralytic ileus from peritonitis after appendicitis. American Journal of Surgery. 1952; 84 :419-428 - 101.
Buchanan L, Tuma F. Postoperative Ileus. StatPearls Publishing; 2022 - 102.
Darwazeh G, Cunningham SC, Kowdley GC. A systematic review of perforated appendicitis and Phlegmon: Interval appendectomy or wait-and-see? The American Surgeon. 2016; 82 :11-15 - 103.
Cheng Y, Xiong X, Lu J, et al. Early versus delayed appendicectomy for appendiceal phlegmon or abscess. Cochrane Database of Systematic Reviews. 2017; 6 :CD011670 - 104.
Ahmed I, Deakin D, Parsons SL. Appendix mass: Do we know how to treat it? Annals of the Royal College of Surgeons of England. 2005; 87 :191-195 - 105.
Akingboye AA, Mahmood F, Zaman S, et al. Early versus delayed (interval) appendicectomy for the management of appendicular abscess and phlegmon: A systematic review and meta-analysis. Langenbeck's Archives of Surgery. 2021; 406 :1341-1351 - 106.
Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: A systematic review and meta-analysis. Annals of Surgery. 2007; 246 :741-748 - 107.
van Amstel P, Sluckin TC, van Amstel T, et al. Management of appendiceal mass and abscess in children; early appendectomy or initial non-operative treatment? A systematic review and meta-analysis. Surgical Endoscopy. 2020; 34 :5234-5249 - 108.
Ward TE, Mangal RK, Stead TS, Ganti L. Hepatic abscess following acute appendicitis. Cureus. 2022; 14 (7):e26867 - 109.
Armstrong T, Dluzewski S, Yu D. Appendicitis with direct fistulation into the liver: A forgotten cause of pyogenic liver abscess. BJR Case Reports. 2020; 6 (4):20200101 - 110.
Saleem MM. Scrotal abscess as a complication of perforated appendicitis: A case report and review of the literature. Cases Journal. 2008; 1 :165 - 111.
Naidoo S, Du Toit R, Bhyat A. Perforated appendicitis presenting as a thigh abscess: A lethal combination. South African Journal of Surgery. 2016; 54 :43 - 112.
Faizi FR, Farzam F. Perforated retrocecal appendicitis presenting with lung abscess-a case report. Radiology Case Reports. 2022; 17 :2754-2758 - 113.
Carter M, Meshkat B, El-Masry S. Epidural abscess secondary to acute appendicitis. BML Case Reports. 2014; 2014 :bcr2014207446 - 114.
Dekkers GH, Dejong CH, Welten RJ. Splenic abscess after appendicitis. Acta Chirurgica Belgica. 2000; 100 :34-36 - 115.
Corder AP. Renal abscess with gas formation secondary to acute appendicitis. British Journal of Urology. 1987; 59 :90 - 116.
Kawamura YJ, Sugamata Y, Yoshino K, et al. Appendico-ileo-vesical fistula. Journal of Gastroenterology. 1998; 33 :868-871 - 117.
McCrystal S, Kong J, Gourlas P, et al. Appendicorectal fistula with a novel solution: First case report and review of the literature. Clinical Case Reports. 2019; 7 :1885-1889 - 118.
Morris-Stiff GJ, Islam KA. Appendico-colic fistula complicating appendicitis in cystic fibrosis. BML Case Reports. 2010; 2010 :bcr0220102714 - 119.
Singh MP, Samaiya A, Sainia TK, Saxena A. Spontaneous appendico-cutaneous fistula: An unusual presentation of retroperitoneal appendicular perforation associated with psoas abscess. Journal of Surgical Case Reports. 2016; 2016 (6):rjw112 - 120.
Singal R, Gupta S, Mittal A, et al. Appendico-cutaneous fistula presenting as a large wound: A rare phenomenon-brief review. Acta Medica Indonesiana. 2012; 44 :53-56 - 121.
Killelea BK, Arkovitz MS. Perforated appendicitis presenting as appendicoumbilical fistula. Pediatric Surgery International. 2006; 22 :286-288 - 122.
Takeda M, Higashi Y, Shoji T, et al. Necrotizing fasciitis caused by a primary appendicocutaneous fistula. Surgery Today. 2012; 42 :781-784 - 123.
Reeves JM, Ip JCY. Not just a simple appendicectomy: Abdominal actinomycosis causing phlegmonous appendicitis. ANZ Journal of Surgery. 2021; 91 :E791-E792 - 124.
Feng W, Du XB, Zhao XF, et al. Risk factors of postoperative adhesive bowel obstruction in children with complicated appendicitis. Pediatric Surgery International. 2021; 37 :745-754 - 125.
Makama JG, Kache SA, Ajah LJ, Ameh EA. Intestinal obstruction caused by appendicitis: A systematic review. Journal of West African College of Surgens. 2017; 7 :94-115 - 126.
Casas MA, Dreifuss NH, Schlottmann F. High-volume center analysis and systematic review of stump appendicitis: Solving the pending issue. European Journal of Trauma and Emergency Surgery. 2022; 48 :1663-1672 - 127.
Pahari S, Shrestha M, Basukala S, et al. Complicated pylephlebitis secondary to perforated appendicitis in a child- a rare case report. Annals of Medicine Surgery (Lond). 2022; 82 :104744 - 128.
Bom WJ, Bolmers MD, Gans SL, et al. Discriminating complicated from uncomplicated appendicitis by ultrasound imaging, computed tomography or magnetic resonance imaging: Systematic review and meta-analysis of diagnostic accuracy. BJS Open. 2021; 5 :zraa030 - 129.
Kim HY, Park JH, Lee YJ, et al. Systematic review and meta-analysis of CT features for differentiating complicated and uncomplicated appendicitis. Radiology. 2018; 287 :104-115 - 130.
Bailey H. The Ochsner-Sherren (delayed) treatment of acute appendicitis: Indications and technique. British Medical Journal. 1930; 1 (3603):140-143 - 131.
Turhan AN, Kapan S, Kütükçü E, et al. Comparison of operative and non operative management of acute appendicitis. Ulusal Travma ve Acil Cerrahi Dergisi. 2009; 15 :459-462 - 132.
Ho CM, Chen Y, Lai HS, et al. Comparison of critical conservative treatment versus emergency operation in children with ruptured appendicitis with tumor formation. Journal of the Formosan Medical Association. 2004; 103 :359-363 - 133.
Simillis C, Symeonides P, Shorthouse AJ, Tekkis PP. A meta-analysis comparing conservative treatment versus acute appendectomy for complicated appendicitis (abscess or phlegmon). Surgery. 2010; 147 :818-829 - 134.
Cunnigaiper ND, Raj P, Ganeshram P, Venkatesan V. Does Ochsner-Sherren regimen still hold true in the management of appendicular mass? Ulusal Travma ve Acil Cerrahi Dergisi. 2010; 16 :43-46 - 135.
Ansaloni L, Catena F, Coccolini F, et al. Surgery versus conservative antibiotic treatment in acute appendicitis: A systematic review and meta-analysis of randomized controlled trials. Digestive Surgery. 2011; 28 :210-221 - 136.
Grossi U, Gallo G, Ortenzi M, et al. Changes in hospital admissions and complications of acute appendicitis during the COVID-19 pandemic: A systematic review and meta-analysis. Health Sciences Review (Oxf). 2022; 3 :100021 - 137.
Köhler F, Müller S, Hendricks A, et al. Changes in appendicitis treatment during the COVID-19 pandemic - a systematic review and meta-analysis. International Journal of Surgery. 2021; 95 :106148 - 138.
Bakker OJ. Should conservative treatment of appendicitis be first line? BMJ. 2012; 344 :e2546 - 139.
Tan APP, Yap TL, Cheong YL, et al. Conservative antibiotic treatment of pediatric acute uncomplicated appendicitis during the COVID-19 pandemic: A prospective comparative cohort study. Pediatric Surgery International. 2022; 39 :60 - 140.
Shay S, Kupietzky A, Weiss DJ, et al. Composite criteria for non-operative Management of Acute non-Complicated Appendicitis Result in low failure rates. World Journal of Surgery. 2022; 46 :69-75 - 141.
Tekin A, Kurtoğlu HC, Can I, Oztan S. Routine interval appendectomy is unnecessary after conservative treatment of appendiceal mass. Colorectal Disease. 2008; 10 :465-468 - 142.
Meshikhes AW. Appendiceal mass: Is interval appendicectomy "something of the past"? World Journal of Gastroenterology. 2011; 17 :2977-2980 - 143.
Sakorafas GH, Mastoraki A, Lappas C, et al. Conservative treatment of acute appendicitis: Heresy or an effective and acceptable alternative to surgery? European Journal of Gastroenterology & Hepatology. 2011; 23 :121-127 - 144.
Dixon MR, Haukoos JS, Park IU, Oliak D, Kumar RR, Arnell TD, et al. An assessment of the severity of recurrent appendicitis. American Journal of Surgery. 2003; 186 :718-722 - 145.
Raveenthiran V, Bharadwaj RA. Mucosa-coring salvage (MU-CO-SAL) Appendicectomy: A useful technique in the Management of Neglected Appendicular Mass. Journal of Indian Association of Pediatric Surgeons. 2020; 25 :239-241 - 146.
Neogi S, Banerjee A, Panda SS, Ratan SK, Narang R. Laparoscopic versus open appendicectomy for complicated appendicitis in children: A systematic review and meta-analysis. Journal of Pediatric Surgery. 2022 Mar; 57 (3):394-405. DOI: 10.1016/j.jpedsurg.2021.07.005 - 147.
Athanasiou C, Lockwood S, Markides GA. Systematic review and meta-analysis of laparoscopic versus open Appendicectomy in adults with complicated appendicitis: An update of the literature. World Journal of Surgery. 2017; 41 (12):3083-3099. DOI: 10.1007/s00268-017-4123-3 - 148.
Zhang S, Du T, Jiang X, Song C. Laparoscopic appendectomy in children with perforated appendicitis: A meta-analysis. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques. 2017 Aug; 27 (4):262-266. DOI: 10.1097/SLE.0000000000000411 - 149.
Wysocki AP, Allen J, Rey-Conde T, North JB. Mortality from acute appendicitis is associated with complex disease and co-morbidity. ANZ Journal of Surgery. 2015; 85 :521-524 - 150.
Goldacre MJ, Duncan ME, Griffith M, et al. Trends in mortality from appendicitis and from gallstone disease in English populations, 1979-2006: Study of multiple-cause coding of deaths. Postgraduate Medical Journal. 2011; 87 :245-250 - 151.
Williams BM, Purcell LN, Varela C, et al. Appendicitis mortality in a resource-limited setting: Issues of access and failure to rescue. The Journal of Surgical Research. 2021; 259 :320-325 - 152.
Dongarwar D, Taylor J, Ajewole V, et al. Trends in appendicitis among pregnant women, the risk for cardiac arrest, and maternal-fetal mortality. World Journal of Surgery. 2020; 44 :3999-4005 - 153.
Vissers RJ, Lennarz WB. Pitfalls in appendicitis. Emergency Medicine Clinics of North America. 2010; 28 :103-118 - 154.
Sosner E, Patlas MN, Chernyak V, et al. Missed acute appendicitis on multidetector computed tomography and magnetic resonance imaging: Legal ramifications, challenges, and avoidance strategies. Current Problems in Diagnostic Radiology. 2017; 46 :360-364 - 155.
Karpelowsky JS, Bickler S, Rode H. Appendicitis--pitfalls and medicolegal implications. South African Medical Journal. 2006; 96 :866-872