Abstract
Autoimmune diseases are a group of chronic inflammatory disorders caused by the imbalance of immune homeostasis and abnormal production of autoantibodies. The etiology of autoimmune diseases involves various factors such as genetic and environmental factors, and the exact pathogenesis remains unclear. The intestinal mucosal immunity including the intestinal epithelial barrier, mucosal immune cells, and innate immune cells cooperatively maintains intestinal immunity against invading pathogens. It has been demonstrated that intestinal mucosal immunity participates in the development of various autoimmune diseases. Dysbiosis of gut microbiota and their metabolite alterations and immune response mediated by intestinal immune cells may be involved in the pathogenesis of systemic lupus erythematosus through multiple mechanisms. When the intestinal mucosal epithelium is damaged, intestinal flora can penetrate the barriers and enter the lamina propria, causing abnormal immune response and inducing the development of Inflammatory Bowel Diseases. Targeting the gut mucosal immune system holds promise for treating autoimmune diseases; therefore, it is necessary to review the role of the gut mucosal immune system in autoimmune diseases and provide guidance for the treatment of autoimmune diseases.
Keywords
- intestinal mucosal immunity
- autoimmune diseases
- gut-associated lymphoid tissue (GALT)
- gut microbiota
- SLE
- IBD
- type I diabetes
1. Introduction
Autoimmune diseases are characterized by a dysregulated immune response leading to excessive and uncontrolled tissue inflammation. Multiple factors including genetic variation, environmental stimuli, and infection have been implicated as contributing factors to persistent inflammation and pathology. Intestinal mucosal immunity is currently considered to be an important factor in regulating the development of autoimmune diseases. In this chapter, we will discuss the compositions of intestinal mucosal immunity and detail the mechanism of intestinal mucosal immunity involved in the pathogenesis of systemic lupus erythematosus (SLE), inflammatory bowel diseases (IBD), and type 1 diabetes mellitus (T1DM), targeting intestinal mucosal immunity for providing new treatment for autoimmune diseases.
2. Summarize
2.1 History of the development of intestinal mucosal immunity
In ancient China, there was a technique to prevent smallpox by grinding the pox scabs of smallpox patients into powder and blowing it into the nasal cavity of healthy people to prevent them from contracting the smallpox virus. This form of immunity was based on protective immunity to the nasal mucosa. With the development of microbiology, Alexandre Besredka [1] developed a method to obtain immune protection by oral administration of bacteria, also known as oral vaccine. This approach also mediated a protective immune response through the mucosa of the digestive tract. With the development of various vaccines, mucosal immunology was gradually developed. These included the discovery of Peyer’s patches at the end of the ileum in the late 17th century [2], the discovery of mucosal tolerance mechanisms in the gut, the identification of secretory and serotype IgA, and the discovery of M cells in the gut in 1982, among others.
2.2 Physiopathology of intestinal mucosal immunity
With the development of various vaccines and modern immunology, intestinal mucosal immunology has been greatly promoted, and mucosal immunity is closely related to both disease and physiology. In terms of pathological regulation, many pathogens including bacteria and viruses infect the body through the mucosal system [3]. Mucosal immunity is also closely related to inflammation and tumors. In addition, mucosal immunity has a very important role in physiological regulation. It is able to interact with commensal bacteria and can mediate the regulation of immunity and neurology, immunity, and metabolism.
2.3 Research methods of intestinal mucosal immunity
The study of intestinal mucosal immunity can be performed using various research methods such as histology and immunohistochemistry [3], which provide information on the distribution and activation status of immune cells within the intestinal mucosa as well as on the expression levels of cytokines and chemokines. Animal models such as germ-free mice, as an effective tool for gut flora research, are in a state of absolute sterility from the embryonic stage, allowing better elucidation of the mechanisms driving gut microbes in many diseases [4]. Combining transcriptomic and microbiomic studies can explore the relationship with host phenotype from the perspectives of host genes and flora, respectively, and can also reveal that key microorganisms regulate host gene expression through correlation analysis [5]. Proteomics enables direct study of protein molecular differences in immune cells between samples, revealing new diagnostic markers and therapeutic targets related to immune disorders [6]. In addition to these approaches, recent advances in single-cell sequencing allow a more detailed understanding of the heterogeneity of the immune cell population within the intestinal mucosa [7]. A large number of in vitro culture and molecular biology techniques are now available for human microbiome studies, which can be used to detect and analyze microbial community composition, species diversity, and effects on human cellular pathways. With the rapid development of modern biomolecular science, we determined the gut microbiota using high-throughput sequencing technology based mainly on 16SrRNA/18SrRNA genes. Through bioinformatics principles and statistical analysis of a large amount of data, we can analyze the diversity of intestinal microorganisms and obtain the type and distribution of intestinal flora to make scientific judgments on the health status of the body through such dynamic changes. In addition, through microbial rRNA gene sequencing, macrogenomics, macrotranscriptomics, and nontargeted metabolomics combined with the core strategy of “whole microbiome association analysis (MWAS)”, we can accurately decode the composition, function, and expression profiles of the flora and uncover key biomarkers to elucidate [8].
In summary, the study of intestinal mucosal immunity is complex and requires the use of multiple research approaches; by combining these approaches, researchers can gain insight into the complex interactions between immune cells and intestinal microbiota in regulating intestinal mucosal immunity and the impact of various interventions on this process.
3. Compositions of intestinal mucosal immunity
The components of intestinal mucosal immunity include gut-associated lymphoid tissue (GALT), the intestinal mucosal epithelium, and gut microbiota (see Figure 1).
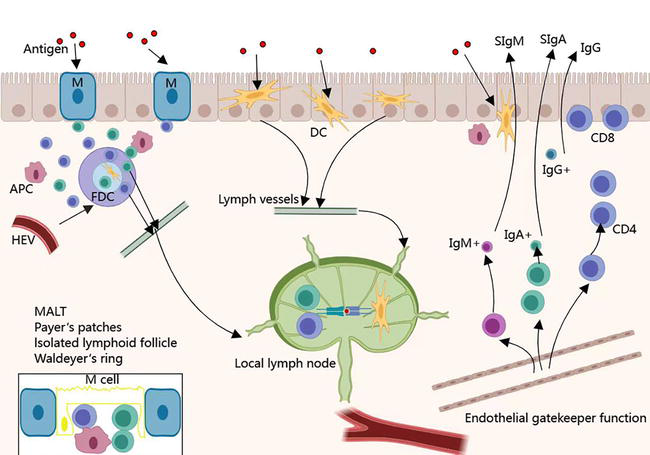
Figure 1.
Compositions of intestinal mucosal immunity.
3.1 Gut-associated lymphoid tissue
GALT composed of Peyer’s patches (PPS), laminar propria lymphocytes, and intraepithelial lymphocytes is an important component of intestinal acquired immunity [9]. As the largest lymphoid tissue in the body, GALT recognizes exogenous and abnormal antigens in a timely manner by uptake, processing, and presentation of antigens. After specific recognition of foreign antigens, GALT promotes the production of cytokines and antibodies to coordinate the immune response. In addition, it activates T lymphocytes and B lymphocytes to establish an effective adaptive immune response and induce a mucosal immune response or immune tolerance [9].
Peyer junction was mainly distributed in the intestinal wall and the inferior mucosa on the opposite side of the attachment margin of the mesentery, which is essential for the induction and initiation of mucosal immunity. In the lamina propria of the mucosa of the GI, there is diffuse lymphoid tissue made of lymphocytes and dendritic cells (DCs). Within the lamina propria, macrophages, mature plasma cells, and sporadic T lymphocytes and B lymphocytes can be found. The role of these cells is to capture and process antigens, which secondly reach regional lymph nodes and start the immune response, eventually followed by the production of immunoglobulin (Ig)A, IgG, and IgM. The myeloid compartment represented by macrophages and DCs is crucial for the maintenance of intestinal tolerance and the activation of T-cell immunity [10, 11]. Intraepithelial lymphocytes (IELs) of the lamina propria are heterogeneous and are represented by a mixed population of T-lymphocytes: mainly differentiation (CD)4(+) cells and some CD8(+) ones. Intraepithelial lymphocytes are a group of smaller lymphocytes distributed among intestinal epithelial cells, mostly located in the columnar epithelium of the intestine and mainly include TCRαβ+ CD8αα + T-cells, TCRγδ+ CD8αα + T-cells, TCRαβ+ CD4+ T-cells and TCRαβ+ CD8αβ + T-cells [12]. They comprise the vast majority of intestinal epithelial cells (IECs) in the small intestine and are essential in maintaining immune homeostasis in the intestinal territory such as keeping the integrity of the gut barrier [13].
3.2 The intestinal mucosal epithelium
The intestinal mucosal epithelium constitutes the physical and chemical barrier of the intestinal mucosal immune system including the epithelial layer and the mucus layer on the surface of epithelial cells [14]. The epithelial layer consists of a single layer of closely connected IECs, composed of a number of cell types such as goblet cells, absorptive enterocytes, enteroendocrine cells, Paneth cells, microfold (M) cells, and tuft cells [14]. IECs separate the internal and external environment of the body and can resist the antigens, toxins, pathogens, and microorganisms in the intestinal lumen. However, IEC also plays an important role in absorbing nutrients present in the intestinal lumen [15]. The mucus layer is the first line of defense in the intestinal mucosal epithelium. Paneth cells are capable of secreting antimicrobial peptides (AMPs) to defend against external pathogens or intestinal bacteria. The mucus layer and AMPs constitute the mucosal barrier to prevent the invasion of symbiotic bacteria.
Additionally, there were scattered intraepithelial lymphocytes (IELs) between closely connected IECs [16]. Approximately 90% of all IEL express T-cell receptors (TCR) [17]. In the small intestine, approximately 1 IEL per 10 IEC [18]. According to different expression profiles of T-cell receptors (TCRs), the TCR+ IELs can be divided into the following two groups: TCRαβ and TCRγδ [12]. Based on the large quantities and special locations, the IELs play an important role in maintaining intestinal immune tolerance and regulating intestinal immunity.
3.3 Gut microbiota
Gut microbiota is a system of microorganisms that resides in the host gut and lives in symbiosis with the host, including bacteria, fungi, and viruses [19]. The gut microbiota and its metabolites maintain the integrity of the intestinal mucosal barrier and participate in the maintenance of the body’s mucosal immune system by regulating innate and adaptive immunity.
Intestinal commensal bacteria promote the development and functional maturation of the host intestinal mucosal immune system. Work in germ-free (GF) mice showed that GF animals have a myriad of intestinal immune defects, including impaired development of GALTs fewer intestinal IgA-secreting plasma cells, smaller PPs, fewer IELs, Treg and Th17 cells [20, 21, 22, 23]. However, most of these deficiencies can be corrected by recolonization with a health-associated mouse commensal microbiota [24, 25]. In normal conditions, intestinal epithelial cells act as a barrier to keep immune cells residing in the intestinal mucosa separate from intestinal microorganisms, thus building a microbial-host symbiosis. In turn, gut microbiota maintains the integrity of the intestinal mechanical barrier. Studies have shown that bacterial metabolites play an important role in maintaining intestinal integrity. Short-chain fatty acids (SCFAs) promote the proliferation of intestinal epithelial cells and promote mucin secretion by goblet cells, which protects the intestinal epithelium from damage by acid and intestinal lumen contents [26]. Furthermore, in vitro experiments have also demonstrated that butyrate can upregulate MUC2 expression by activating the MUC2 promoter and altering histone modifications in this region [27]. Additionally, bacteria components such as lipopolysaccharide (LPS) and flagellin can be recognized by pattern recognition receptors IECs, which promote cell proliferation and the production of cytokines, antimicrobial peptides, and mucus [28].
Microbiota plays another important role in the intestinal mucosal immune system, that is, the intestinal commensal bacteria involved in immune response and immune modulation. Many studies have shown that dysbiosis of microbiota increases host susceptibility to a variety of immune, inflammatory, and allergic diseases, which may be because of the gut microbiota involved in CD4+ T-cell differentiation via different pathways as well as in the induction of sIgA [29, 30, 31]. Specifically, it can be divided into the following points: 1) Microbiota can affect Th17 differentiation and participate in the mucosal immune system defense against pathogens. For example, after SFB is colonized, naive CD4 T-cells migrate olites can induce the differentiation of Treg cells, participate in the immune reg to the small intestine and differentiate into IL-17A-producing Th17 cells, whose products stimulate the production of antimicrobial peptides by IECs [32]. 2) Microbiota and its metabulation and maintenance of immune homeostasis together with Th17 cells [31]. 3) Microbiota can regulate the intestinal T follicular helper cells, which can participate in the production of high-affinity antibodies by B-cells and it is thought to be the chief cell regulating B-cells in germinal centers. As the SFB colonized in IL-21R-deficient mice, the number of IgA plasmablasts and plasma cells decreased significantly [33]. 4) Microbiota can affect the accumulation of sIgA-producing plasma cells and also affect the diversity of IgA in the lymphocyte tissues of the gut [34].
Therefore, the interaction between the microbiota and the intestinal immune system is essential to maintain intramucosal homeostasis. However, ecological dysbiosis can lead to intestinal diseases when the balanced intestinal microbial community is altered.
4. Intestinal mucosal immunity and autoimmune diseases
There is a close yet complex link between intestinal flora and immune-related diseases. Dysbiosis of intestinal flora plays an important role in the development of immune-related diseases including SLE, IBD, and T1DM (Figure 2).
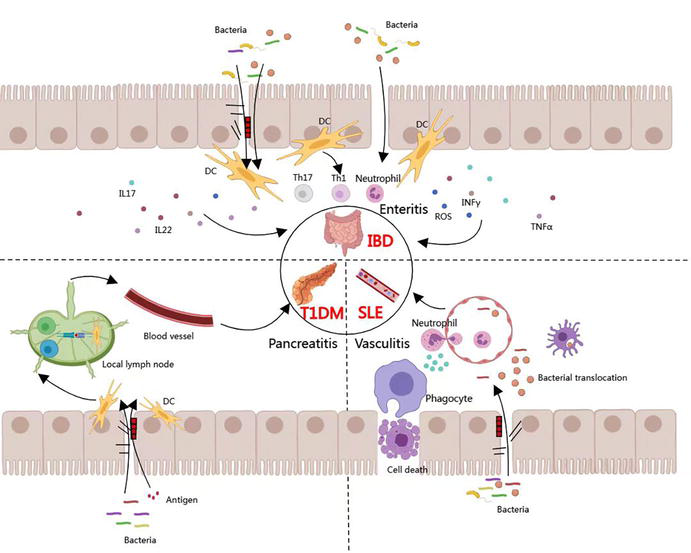
Figure 2.
Association between intestinal mucosal immunity and immunologic diseases.
4.1 Systemic lupus erythematosus
SLE is an autoimmune disease characterized by the presence of nuclear autoantibodies and complex immune inflammation involving multiple organs. The pathogenesis is not fully understood. Some factors such as molecular genetics, epigenetics, immunomodulation, ethnicity, possible environmental influences (ultraviolet light, drugs, and infection) and sex hormones are associated with its occurrence [35]. Gut microbiota and their metabolites have been proposed to be involved in SLE development and progression through intestinal mucosal immunity [36].
Increasing evidence supports that dysbiosis of gut microbiota is associated with lupus pathogenesis. The number of non-pathogenic bacteria such as Bifidobacterium and Bacteroides fragilis in the intestine of SLE patients was significantly reduced compared with normal controls, whereas the number of conditionally pathogenic bacteria such as Ruminococcus gnavus and Enterococcus gallinarum was significantly increased compared with the normal group [36]. It has been indicated that gut microbiota metabolites such as SCFAs amino acids and lipids are correlated with SLE. Most of the SCFA that exist in the gut are acetate, propionate, and butyrate [37]. The immunoregulatory functions of SCFA range from anti-inflammatory, T cell, and epigenetic pathways. Bacteroides and Negativicutes produce propionate through the succinate pathway. However, butyrate was mainly produced by phylum Firmicutes via the acetate CoA-transferase pathway [38]. Butyrate decreases permeability by accelerating the assembly of tight junctions via the activation AMPK [39]. Bifidobacterium species increase gut barrier integrity by producing acetate, which increases the expression of the tight junction gene occludin [40]. Because most butyrate-producing bacteria belong to the Firmicutes phylum, inflammation and impaired gut barrier integrity may be induced by the decreased relative abundance of Firmicutes in SLE patients [36]. Changes in intestinal flora associated with changes in lupus disease, such as the abundance of Streptococcus, Campylobacter, Veillonella, Clostridiacae, and Lachnospiraceae, were positively correlated with SLE disease activity. The abundance of Bifidobacterium was negatively correlated with lupus activity. In addition [41], the intestinal flora of SLE patients with lupus nephritis and gastrointestinal damage showed a decrease in the thick-walled phylum, an increase in the Aspergillus phylum, and a relative increase in the Aspergillus phylum, Enterobacteriaceae, and Shigella coli.
Gut microbiota influences SLE through different mechanisms. First, the abnormal gut microbiota translocation results in what is known as the “leaky gut,” triggering the systemic autoimmune response [36]. The imbalance of gut microbiota can compromise intestinal permeability, allowing the passage of antigens and bacteria in the lumen to the blood circulation, which causes leaky gut syndromes that exacerbate lupus immunopathogenesis [42]. It has been reported that toll-like receptors (TLRs) contribute to lupus pathogenesis by sensing harmful bacteria coming from gut microbiota through microbial translocation, especially in the presence of a leaky gut. Activation of TLR4 with LPS inducing the release of CD14 from monocytes contributes to the pathogenesis of SLE and exacerbates its development [43]. Second, interference with immune cells in the lamina propria leads to the development of SLE. Aberrant monocyte/macrophage surface markers were expressed in cells from SLE patients, including Fcγ receptors, ICAM-1 (intercellular adhesion molecule-1, CD54), CD40, MHC II, type-1 interferon-stimulated genes, and sialoadhesin (Siglec-1,CD169) [44]. Besides the deregulations of monocyte/macrophage surface markers, macrophages in SLE patients also have a defect in phagocytosis. Ineffective clearance of dying cells and debris by macrophages may provide a source of autoantigens for the development of autoantibodies in SLE disease [45]. TLRs are a type of cell transmembrane signal transduction protein in the innate immune system, which recognizes microbial-related molecular patterns to trigger different immune responses. MYD88 is the main linker molecule of the TLR signal transduction pathway. Most studies suggest that the TLR/MYD88 signaling pathway plays an important role in restricting the penetration of the intestinal microbiome and preventing mucosal immune regulation disorders, and it is essential for maintaining a normal intestinal mucosal barrier and regulating intestinal homeostasis [46]. LPS mostly coming from the intestinal lumen is recognized by TLR4 and the interaction between them has been proven to promote the inflammatory response and exacerbate the development of SLE [47]. Studies found that the high level of plasma LPS in patients with SLE caused by the impaired intestinal mucosal barrier was positively correlated with the level of serum anti-double-stranded DNA antibodies, which suggested that the increased intestinal permeability was beneficial to LPS in order to penetrate the intestinal epithelium and translocate into the tissue to promote the progression of disease [48]. Moreover, altered inflammatory cytokine production from monocytes/macrophages has also been found in SLE patients. Elevated monocyte counts, increased CD16 expression, and IL-6 production in monocytes were found in SLE patients. Plasma sCD14 and IL-6 cytokines released by monocytes in response to LPS are increased in lupus patients relative to controls [49]. Macrophage-mediated maintenance of tolerance and prevention of proinflammatory responses to TLR ligands at the intestinal mucosal site is important for mucosal immunity. Altered TLR-mediated innate immune responses in intestinal macrophages may play a key role in SLE disease pathogenesis [50]. Finally, The cross-reactivity between certain bacteria antigens with specific autoantibodies has been recognized as the main factor of leaky gut syndrome in lupus. Autoantibodies to the 60 kDa Ro protein are common in SLE patients. It was found early that the lupus autoantigen Ro60 cross-reacted with the Ebstein-Barr virus nuclear antigen-1 (EBNA-1), suggesting that lupus humoral autoimmunity was initiated via molecular mimicry of EBNA-1 and Ro60 antigens [51]. Colonization of Bacteroides thetaiotaomicron in lupus mice was found to enhance the expression of Ro60 antigen followed by deposition of immune complex causing lupus nephritis [41]. Gut microbiota maintains a symbiotic relationship with the host. It uses the energy and sources of the host to ensure its growth and releases metabolites, which in turn influence the host’s metabolism.
Impaired composition and function of gut microbiota have been associated with several autoimmune diseases including SLE. These mechanisms include leaky gastrointestinal tract disturbing its equilibrium, crossreactivity of microbial proteins with self-antigens, and dysregulation of both innate and adaptive immunity. These alterations lead to self-tolerance breakdown and autoantibodies production. Therefore, it is important to decipher the commensal bacteria profiles associated with SLE because these may guide the scientific community to a better understanding of the disease.
4.2 Inflammatory bowel diseases
IBD, which mainly includes Crohn’s disease (CD) and ulcerative colitis (UC), are chronic, nonspecific, relapsing, and inflammatory disease mainly involving the gastrointestinal tract [52]. The global burden of IBD remains a persistent health problem. The prevalence of IBD in Europe, North America, and other Western countries exceeds 0.3% and is increasing in many newly industrialized countries [53, 54]. The etiology of IBD is not yet fully understood, and it has been proven to be related to complex factors such as genetics, environment, intestinal microbes, and immunity [55].
In recent years, studies have suggested that intestinal mucosal immunity plays a pivotal role. Several clinical studies have shown that UC and CD patients have different degrees of intestinal flora dysbiosis with reduced intestinal flora diversity, reduced intestinal probiotics, and increased pathogenic bacteria [56]. Sequencing of intestinal flora revealed that probiotics such as Bifidobacterium and Lactobacillus were significantly reduced and pathogenic bacteria such as Enterococcus and Enterobacteriaceae were increased in the intestinal flora of UC patients compared with healthy adults, whereas probiotics such as Lactobacillus, Bacteroides, and Rumenococcus were reduced and Actinobacillus, Aspergillus, and Enterobacteriaceae were increased in CD patients. It was found that the site of lesion, disease activity, and duration of disease in IBD patients have an effect on their intestinal flora. Besides, the disease activity of IBD patients also had an effect on the intestinal flora with an increase in disease activity index in CD patients associated with an increase in Enterobacteriaceae, but no association was found between disease activity index and intestinal flora in UC patients [57]. Patients with IBD not only have dysregulated numbers and types of intestinal flora but also abnormalities in the function of intestinal flora [58]. A study in a mouse model of colitis found that butyrate, one of the SCFA, maintains Th17/Treg balance in the gut and exerts anti-inflammatory effects [59]. Certain intestinal bacteria such as F. saccharivorans may also exert their anti-inflammatory effects by producing SCFA, whereas butyrate-producing flora such as Roseburia hominis (R. hominis) and E. pumilus are reduced in patients with UC and their reduction may have contributed to the overexpression of proinflammatory factors in human intestinal cells, which can cause inflammation [60]. In addition, smoking exacerbates the symptoms of enteritis in CD patients [61]. External factors such as smoking, diet, medications, and emotions act on the intestinal flora, causing a decrease in the abundance of SCFAs-producing organisms (e.g., B. thicketi) and an increase in the abundance of pathogenic organisms (e.g., Aspergillus, Bacteroidetes), which can contribute to the development of intestinal inflammation in susceptible individuals [55].
The pathogenesis of IBD has not been fully elucidated, and a large number of studies have found that the intestinal flora plays an important role and that intestinal mucosal immune disorders may be the initiating factor in the pathogenesis of IBD. Barrier disruption of the intestinal mucosal epithelium is an important pathogenetic feature of IBD. When the epithelium is damaged, the mechanical barrier permeability is greatly increased, and intestinal lumen such as macromolecular proteins and pathogenic bacteria can enter the lamina propria through the broken mechanical barrier, inducing the development of IBD [62]. With the gradual development of high-throughput assays, IncRNA, as a new molecule in biology, has been shown to activate intestinal inflammatory genes, damage epithelial cells, and disrupt intercellular tight junctions leading to an abnormal increase in the permeability of the mechanical barrier of the intestinal mucosa and affecting the intestinal barrier, which is an important precursor alteration in the pathogenesis of IBD [63]. When IBD occurs, the mucus barrier function is absent. It has been shown that in patients with active UC and animal models, intestinal mucus granules are reduced and mucus is thinned, allowing microorganisms to be exposed to the surface of intestinal mucosal epithelial cells [63]; whereas in patients with CD, the production of mucus is instead increased, which is considered to be a result of the disruption of the integrity of the mucus network by the production of sulfides by a number of specific bacteria in the intestinal mucus, which in turn increases the host’s contact with microorganisms and toxins, and induces inflammatory responses [64]. Changes in the intestinal microbiota are accompanied by the thinning of the intestinal mucus layer leading to barrier rupture, epithelial defects, and displacement of intestinal flora across the barrier to induce DC and macrophage activation, which induces inflammatory CD4T cell infiltration into intestinal tissues [65]. Normal intestinal immunity protects against pathogen invasion but a sustained, abnormal immune response can damage the intestinal wall [66]. Patients with IBD have been found to have abnormalities in both innate and adaptive immunity, with differences in the immunologic profile of UC and CD. Patients with IBD have increased levels of pro-inflammatory Th1 and Th17 cells. This infiltration is accompanied by an increase in the number of Th2 cells and a deficit in the number of immunosuppressive cells (e.g., Tregs) [67] . Inflammatory T cells direct the function of cells with innate immunity such as epithelial cells, fibroblasts, and phagocytes, thus stimulating a sustained hyperreactivity to microbial antigens and causing tissue damage and chronic intestinal inflammation [68]. Therefore, when the physical, chemical, and mucus barriers are compromised, intestinal flora can penetrate the barriers and enter the lamina propria, inducing an abnormal immune response.
Because of the adverse effects and ineffectiveness of some of the standardized therapies for IBD, the search for new and effective therapies has always been a goal pursued by researchers and clinicians. Currently, researchers are committed to treating IBD by restoring gut microbial homeostasis and improving intestinal inflammation through the use of probiotics, prebiotics, synbiotics, and fecal microbial transplants as complementary and alternative medications, among others. Fecal microbial transplantation is the process of transplanting functional flora from the feces of a healthy population into the gastrointestinal tract of a patient. The use of FMT in IBD patients is expected to treat the disease by restoring gut microbial homeostasis in patients. Currently, four clinical randomized controlled studies of FMT for the treatment of UC have been reported, three of which concluded that FMT was effective in inducing remission of UC [69, 70, 71]. A recent network meta-analysis in UC has described that when compared with available targeted pharmacotherapies, fecal microbiota transplantation (FMT) has a comparable effect in inducing clinical remission, clinical response, and endoscopic remission [72]. Data from the US clinicaltrial.gov website show that several RCTs of FMT for CD are underway but no relevant RCTs have been reported. One recent systematic review of FMT for IBD, which counted the results of 11 studies in CD, showed a 50.5% remission rate of FMT in CD (42/83) [73]. In a meta-analysis of 12 studies, FMT was associated with clinical remission and clinical response in 62% and 79% of patients with CD, respectively [74]. This shows that FMT has some potential for the treatment of IBD and it may provide a new therapeutic pathway for patients with IBD, especially for those who have failed to be treated with traditional methods but more clinical studies need to be conducted to confirm this.
4.3 Type I diabetes mellitus
Type 1 diabetes mellitus (T1DM), also known as insulin-dependent diabetes mellitus, usually starts in adolescence. T1DM patients have absolute insulin deficiency due to autoimmune destruction of pancreatic beta cells so exogenous insulin is needed to control blood glucose [75]. In recent years, due to rapid economic development and unhealthy lifestyle, type I diabetes has become a metabolic disease that seriously threatens human life expectancy.
Because of the many factors involved in the pathogenesis and the close relationship between them, the etiology of the disease is still not fully understood. Recent studies have found that the intestinal microbial diversity and abundance are lower in TDM patients than in healthy controls, with lower abundance of both the Actinobacteria and thick-walled bacteria phylum, lower abundance of Lactobacillus, Bifidobacterium, Clostridium/Proctobacteria and Prevotella, and higher abundance of the Mycobacterium phylum [76]. Follow-up studies also found that in quantitative experiments of intestinal metabolites, the metabolic production of intestinal butyrate was significantly different in normal healthy individuals compared with type 1 diabetics, and levels of butyrate-producing bacteria were significantly lower in type I diabetics compared with normal healthy individuals based on quantitative comparisons of intestinal flora [77].
The intestinal microbiota may act by influencing intestinal permeability, molecular mimicry, and modulation of the innate and adaptive immune systems. Studies have shown that when the intestinal barrier is compromised, pancreatic-draining lymph node T cells, particularly diabetic-derived CD8+ T cells, will be activated and will proliferate, promoting insulitis [77]. In addition, many other studies have shown that changes in certain microorganisms such as Clostridium perfringens, invisible Dialister, Gemella sanguinis, and Bifidobacterium longum are associated with impaired intestinal integrity and increased risk of T1DM [78]. When intestinal permeability is increased, intestinal toxins, food antigens, and infection factors can transit from the gastrointestinal lumen to the intestinal mucosal components and eventually to the pancreatic lymph nodes, inducing or exacerbating TID. A recent proteomic analysis also showed that the intestinal flora associated with host proteins related to the maintenance of mucus barrier function and microvillus adhesion is depleted in patients with new-onset T1DM [79]. In addition, children at high risk for T1DM have increased intestinal permeability and are associated with altered intestinal flora [80]. Therefore, the intestinal flora and its metabolites may be able to influence the development of T1DM by altering the barrier function of the intestine. Microbial peptide mimics produced by Clostridium perfringens to mimic sequences in the insulin b chain and trigger or participate in the immune response to T1DM development. Flavobacterium, Bacillus cereus, and Enterobacter mori LMG 25706 (Leptotrichia goodfellowii, Flavobacteriia bacterium, Bacillus cereus, and Enterobacter mori LMG 25706) also possess diabetic IGRP206–214 homologous peptide that can induce or accelerate T1DM through molecular mimicry [81]. The interaction between gut microbes and immunity is also crucial in the development and pathogenesis of T1DM. For example, studies in animals lacking myeloid differentiation primary response protein 88 (MyD88) have highlighted the link between gut flora-induced alterations in innate immunity and the risk of T1DM. MyD88 induces toll-like receptors in the intestinal flora, triggers different toll-like receptors, and induces pre- and anti-diabetic signals to promote cellular responses to LPS [75, 82]. A study by Gülden et al. revealed novel innate immune pathways influenced by gut flora in T1DM development. They found that knockdown of the β-interferon TIR structural region articulation protein (TIR-domain-containing adaptor inducing interferon-β, TRIF), another key articulator protein downstream of the TLR, protected NOD mice from diabetes. Importantly, different gut flora characteristics were found in TRIF-deficient NOD mice compared with wild-type NOD mice, suggesting that the protective effect of TRIF deficiency is mediated by altered gut flora [83]. Some specific intestinal bacteria have the ability to regulate T cell subsets and functions. Listeria monocytogenes can induce Th1 responses, whereas segmented filamentous bacteria can enhance Th17 responses. Altered schedule flora and Clostridium perfringens consortia have the ability to induce regulatory T cells [64, 84]. In addition, altered intestinal flora can increase the number of type 1 regulatory T (Tr1) cells in the intestine [85]. These Tr1 cells can migrate to the periphery, inhibit the activation of effector T cells, and reduce the incidence of diabetes.
In addition, the gut microbiome also plays a key role in the development of certain subpopulations of innate T-cells such as mucosal associated invariant T (MAIT) cells. Mucosal associated invariant T (MAIT) cells are innate T-like cells that recognize derivatives of bacterial riboflavin metabolites presented by MHC-Ib associated protein 1 (MR1) molecules and are important effector cells in mucosal immunity [86]. Upon activation, MAIT cells produce several proinflammatory cytokines such as IFN-γ and IL-17A and exhibit cytotoxic effects on cells infected with certain pathogens [87]. In recent years, changes in circulating MAIT compartments such as T1DM have been observed in a variety of autoimmune diseases. In the intestinal mucosa, MAIT cells may play a protective role by producing IL-17A and IL-22, two key cytokines in intestinal homeostasis [88, 89, 90, 91]. This increased pathogenic response is associated with loss of intestinal integrity, decreased expression of tight junction proteins, and abnormal mucus distribution. Thus, the gut microbiota may be a key regulator of T1DM pathogenesis.
5. Treatment progress of autoimmune diseases based on intestinal mucosal immunity
Traditional treatment methods have played a significant role in controlling and managing autoimmune diseases primarily including glucocorticoids, immunosuppressants, and intravenous immunoglobulins. With in-depth research on the mucosal immune system and autoimmune diseases, emerging treatment approaches are continually emerging, providing new options for improving treatment outcomes and reducing side effects.
Interventions targeting key regulatory molecules of mucosal immune responses and suppressing or enhancing specific immune pathways or signaling molecules can precisely modulate the activity of the immune system [92]. In mucosal immune responses, prominent proinflammatory cytokines and growth factors include IL-1β, interferon-gamma, TNF-alpha (TNF-α), and IL-6. Anifrolumab targets and inhibits signaling through the type I interferon receptor subunit 1 and has shown efficacy in SLE evidenced by improvements in various clinical outcomes including mucocutaneous and musculoskeletal manifestations, lower flare rates, relapse rates, and successful tapering from glucocorticoids to ≤7.5 mg/day [93]. Anti-tumor necrosis TNF-α antibody drugs such as infliximab, adalimumab, and certolizumab pegol have demonstrated significant clinical success and are widely used as first-line therapy for IBD [42].
Enhancement or modulation of mucosal immune responses can be achieved by introducing modified immune cells into the patient’s body. The most commonly used approach currently is the fusion of T cells with antigen-specific chimeric molecules known as chimeric antigen receptor T-cell (CAR-T) therapy. In 2021, Dimitrios M et al. reported the data on the use of anti-CD19 CAR-T cells in patients with refractory SLE, showing rapid clinical remission and no significant adverse reactions accompanied by sustained depletion of circulating B lymphocytes and rapid disappearance of serum anti-DNA antibodies [94]. Toxicities associated with this therapy mainly include cytokine release syndrome, which can be life-threatening; but in most cases, effective specific strategies including the use of monoclonal antibodies blocking IL-6 activity can be employed to mitigate the release of proinflammatory cytokines. CAR-T cell therapy represents an interesting and promising approach for SLE treatment but further randomized controlled trials are needed to evaluate its efficacy.
The structure and function of the gut microbiome can influence the balance of the mucosal immune system. Microbiota-based therapies include fecal microbiota transplantation, probiotics, prebiotics, and postbiotics, with the basic principle of introducing or promoting potentially beneficial microorganisms in patients. They have been extensively studied in diseases such as IBD and type 1 diabetes [76]. For example, Butzner JD et al. found that butyrate enemas effectively treated ulcerative colitis and UC patients, whereas Sun J et al. discovered that injection of butyrate into spontaneously diabetic NOD mice controlled pancreatic inflammation by regulating cathelicidin-related antimicrobial peptide production, thereby suppressing the development of autoimmune diabetes [95]. Although microbiota-based therapies are still in the early stages, ongoing research and clinical trials suggest that this approach may be the most effective method for developing IBD treatments.
The relationship between the mucosal immune system and autoimmune diseases is complex and diverse. In-depth understanding of abnormalities in the mucosal immune system and their interaction with specific diseases is of significant importance in developing novel therapeutic approaches for these diseases. Further research on the association between the mucosal immune system and autoimmune diseases contributes to a better understanding of the underlying mechanisms and provides new insights and strategies for treatment and prevention.
6. Conclusion
The role of intestinal mucosal immunity in autoimmune diseases has received increasing attention. Reduced diversity and abnormal strain distribution of intestinal flora have been detected in patients with a variety of autoimmune diseases, and the possible mechanisms involved in immune disorders include: translocation and molecular mimicry of the flora, dysregulation of flora, SCFAs inducing immune imbalance, epitope expansion, and bystander activation. The relevance of intestinal mucosal immunity to autoimmune diseases was further investigated by combining a preclinical model (a germ-free mouse model) and host multi-omics characterization approaches (e.g., 16SrRNA/18SrRNA genes, high-throughput sequencing, macrogenomics, macrotranscriptomics and nontargeted metabolomics, transcriptomics). This provides a possibility to develop interventions based on intestinal flora for the treatment of autoimmune diseases.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (project no. 81300583), Science and Technology Innovation Project of Fujian Province (project no. 2019Y9043), Key Project of Social Development of Fujian Province of China (project no. 2019Y0069), Key Project of Social Development of Fujian Province of China (project no. 2023Y0068) and Foundation of The 900th Hospital of Joint Logistic Team (project no. 2021MS15, 2021JQ10, 2022MS30, 2022ZD05, 2023ON02).
References
- 1.
Rietschel ET, Cavaillon JM. Richard Pfeiffer and Alexandre Besredka: Creators of the concept of endotoxin and anti-endotoxin. Microbes and Infection. 2003; 5 (15):1407-1414. DOI: 10.1016/j.micinf.2003.10.003 - 2.
CDC. Update: Mass vaccination with oral poliovirus vaccine--Asia and Europe, 1996. MMWR. Morbidity and Mortality Weekly Report. 1996; 45 (42):911-914 - 3.
Perez-Lopez A, Behnsen J, Nuccio SP, et al. Mucosal immunity to pathogenic intestinal bacteria. Nature Reviews. Immunology. 2016; 16 (3):135-148. DOI: 10.1038/nri.2015.17 - 4.
Bamias G, Arseneau KO, Cominelli F. Mouse models of inflammatory bowel disease for investigating mucosal immunity in the intestine. Current Opinion in Gastroenterology. 2017; 33 (6):411-416. DOI: 10.1097/MOG.0000000000000402 - 5.
James KR, Elmentaite R, Teichmann SA, et al. Redefining intestinal immunity with single-cell transcriptomics. Mucosal Immunology. 2022; 15 (4):531-541. DOI: 10.1038/s41385-021-00470-y - 6.
Wen J, Niu X, Chen S, et al. Chitosan oligosaccharide improves the mucosal immunity of small intestine through activating SIgA production in mice: Proteomic analysis. International Immunopharmacology. 2022; 109 :108826. DOI: 10.1016/j.intimp.2022.108826 - 7.
Lemme-Dumit JM. mSphere of influence: Organoids and single-cell sequencing, a powerful combination to uncover epithelial and immune cell interactions in the human gut environment. mSphere. 2020; 5 (4):e00722-20. DOI: 10.1128/mSphere.00722-20 - 8.
Kanangat S, Skaljic I. Microbiome analysis, the immune response and transplantation in the era of next generation sequencing. Human Immunology. 2021; 82 (11):883-901. DOI: 10.1016/j.humimm.2021.07.009 - 9.
Morbe UM, Jorgensen PB, Fenton TM, et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunology. 2021; 14 (4):793-802. DOI: 10.1038/s41385-021-00389-4 - 10.
Stagg AJ. Intestinal dendritic cells in health and gut inflammation. Frontiers in Immunology. 2018; 9 :2883. DOI: 10.3389/fimmu.2018.02883 - 11.
Sun T, Nguyen A, Gommerman JL. Dendritic cell subsets in intestinal immunity and inflammation. Journal of Immunology. 2020; 204 (5):1075-1083. DOI: 10.4049/jimmunol.1900710 - 12.
Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nature Reviews. Immunology. 2011; 11 (7):445-456. DOI: 10.1038/nri3007 - 13.
Han J, Liu N, Jin W, et al. TGF-beta controls development of TCRgammadelta(+)CD8alphaalpha(+) intestinal intraepithelial lymphocytes. Cell Discovery. 2023; 9 (1):52. DOI: 10.1038/s41421-023-00542-2 - 14.
Shi N, Li N, Duan X, et al. Interaction between the gut microbiome and mucosal immune system. Military Medical Research. 2017; 4 :14. DOI: 10.1186/s40779-017-0122-9 - 15.
Gunther C, Neumann H, Neurath MF, et al. Apoptosis, necrosis and necroptosis: Cell death regulation in the intestinal epithelium. Gut. 2013; 62 (7):1062-1071. DOI: 10.1136/gutjnl-2011-301364 - 16.
Brandtzaeg P, Pabst R. Let’s go mucosal: Communication on slippery ground. Trends in Immunology. 2004; 25 (11):570-577. DOI: 10.1016/j.it.2004.09.005 - 17.
Olivares-Villagomez D, Van Kaer L. Intestinal intraepithelial lymphocytes: Sentinels of the mucosal barrier. Trends in Immunology. 2018; 39 (4):264-275. DOI: 10.1016/j.it.2017.11.003 - 18.
Ma H, Qiu Y, Yang H. Intestinal intraepithelial lymphocytes: Maintainers of intestinal immune tolerance and regulators of intestinal immunity. Journal of Leukocyte Biology. 2021; 109 (2):339-347. DOI: 10.1002/JLB.3RU0220-111 - 19.
Kc D, Sumner R, Lippmann S. Gut microbiota and health. Postgraduate Medicine. 2020; 132 (3):274. DOI: 10.1080/00325481.2019.1662711 - 20.
Bandeira A, Mota-Santos T, Itohara S, et al. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. The Journal of Experimental Medicine. 1990; 172 (1):239-244. DOI: 10.1084/jem.172.1.239 - 21.
Ostman S, Rask C, Wold AE, et al. Impaired regulatory T cell function in germ-free mice. European Journal of Immunology. 2006; 36 (9):2336-2346. DOI: 10.1002/eji.200535244 - 22.
Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Seminars in Immunology. 2007; 19 (2):59-69. DOI: 10.1016/j.smim.2006.10.002 - 23.
Pickard JM, Zeng MY, Caruso R, et al. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunological Reviews. 2017; 279 (1):70-89. DOI: 10.1111/imr.12567 - 24.
Hapfelmeier S, Lawson MA, Slack E, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010; 328 (5986):1705-1709. DOI: 10.1126/science.1188454 - 25.
Chung H, Pamp SJ, Hill JA, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012; 149 (7):1578-1593. DOI: 10.1016/j.cell.2012.04.037 - 26.
Finnie IA, Dwarakanath AD, Taylor BA, et al. Colonic mucin synthesis is increased by sodium butyrate. Gut. 1995; 36 (1):93-99. DOI: 10.1136/gut.36.1.93 - 27.
Burger-van PN, Vincent A, Puiman PJ, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. The Biochemical Journal. 2009; 420 (2):211-219. DOI: 10.1042/BJ20082222 - 28.
Kayama H, Okumura R, Takeda K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annual Review of Immunology. 2020; 38 :23-48. DOI: 10.1146/annurev-immunol-070119-115104 - 29.
Chiu CY, Chan YL, Tsai MH, et al. Gut microbial dysbiosis is associated with allergen-specific IgE responses in young children with airway allergies. World Allergy Organization Journal. 2019; 12 (3):100021. DOI: 10.1016/j.waojou.2019.100021 - 30.
Meisel M, Mayassi T, Fehlner-Peach H, et al. Interleukin-15 promotes intestinal dysbiosis with butyrate deficiency associated with increased susceptibility to colitis. The ISME Journal. 2017; 11 (1):15-30. DOI: 10.1038/ismej.2016.114 - 31.
Wang L, Zhu L, Qin S. Gut microbiota modulation on intestinal mucosal adaptive immunity. Journal of Immunology Research. 2019; 2019 :4735040. DOI: 10.1155/2019/4735040 - 32.
Weaver CT, Elson CO, Fouser LA, et al. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annual Review of Pathology. 2013; 8 :477-512. DOI: 10.1146/annurev-pathol-011110-130318 - 33.
Cho H, Jaime H, de Oliveira RP, et al. Defective IgA response to atypical intestinal commensals in IL-21 receptor deficiency reshapes immune cell homeostasis and mucosal immunity. Mucosal Immunology. 2019; 12 (1):85-96. DOI: 10.1038/s41385-018-0056-x - 34.
Moon C, Baldridge MT, Wallace MA, et al. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015; 521 (7550):90-93. DOI: 10.1038/nature14139 - 35.
Brown EM, Kenny DJ, Xavier RJ. Gut microbiota regulation of T cells during inflammation and autoimmunity. Annual Review of Immunology. 2019; 37 :599-624. DOI: 10.1146/annurev-immunol-042718-041841 - 36.
Ma L, Morel L. Loss of gut barrier integrity In lupus. Frontiers in Immunology. 2022; 13 :919792. DOI: 10.3389/fimmu.2022.919792 - 37.
Barbhaiya M, Costenbader KH. Environmental exposures and the development of systemic lupus erythematosus. Current Opinion in Rheumatology. 2016; 28 (5):497-505. DOI: 10.1097/BOR.0000000000000318 - 38.
Wu Y, Wang CZ, Wan JY, et al. Dissecting the interplay mechanism between epigenetics and gut microbiota: Health maintenance and disease prevention. International Journal of Molecular Sciences. 2021; 22 (13):6933. DOI: 10.3390/ijms22136933 - 39.
Peng L, Li ZR, Green RS, et al. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. The Journal of Nutrition. 2009; 139 (9):1619-1625. DOI: 10.3945/jn.109.104638 - 40.
Hsieh CY, Osaka T, Moriyama E, et al. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiological Reports. 2015; 3 (3):e12327. DOI: 10.14814/phy2.12327 - 41.
Mohd R, Chin S-F, Shaharir SS, et al. Involvement of gut microbiota in SLE and lupus nephritis. Biomedicine. 2023; 11 (3):653. DOI: 10.3390/biomedicines11030653 - 42.
Yaigoub H, Fath N, Tirichen H, et al. Bidirectional crosstalk between dysbiotic gut microbiota and systemic lupus erythematosus: What is new in therapeutic approaches? Clinical Immunology. 2022; 244 :109109. DOI: 10.1016/j.clim.2022.109109 - 43.
Mu Q , Kirby J, Reilly CM, et al. Leaky gut As a danger signal for autoimmune diseases. Frontiers in Immunology. 2017; 8 :598. DOI: 10.3389/fimmu.2017.00598 - 44.
Kyttaris VC, Juang YT, Tsokos GC. Immune cells and cytokines in systemic lupus erythematosus: An update. Current Opinion in Rheumatology. 2005; 17 (5):518-522. DOI: 10.1097/01.bor.0000170479.01451.ab - 45.
Zhou Z, Ding M, Huang L, et al. Toll-like receptor-mediated immune responses in intestinal macrophages; implications for mucosal immunity and autoimmune diseases. Clinical Immunology. 2016; 173 :81-86. DOI: 10.1016/j.clim.2016.09.005 - 46.
Kumar V. Corrigendum to ‘Toll-like receptors in immunity and inflammatory diseases: Past, present, and future’ [international Immunopharmacology 59 (2018) 391-412]. International Immunopharmacology. 2018; 62 :338. DOI: 10.1016/j.intimp.2018.06.044 - 47.
Mu Q , Zhang H, Luo XM. SLE: Another autoimmune disorder influenced by microbes and diet? Frontiers in Immunology. 2015; 6 :608. DOI: 10.3389/fimmu.2015.00608 - 48.
An J, Liu Y, Wang Y, et al. The role of intestinal mucosal barrier in autoimmune disease: A potential target. Frontiers in Immunology. 2022; 13 :871713. DOI: 10.3389/fimmu.2022.871713 - 49.
Pan F, Tang W, Zhou Z, et al. Intestinal macrophages in mucosal immunity and their role in systemic lupus erythematosus disease. Lupus. 2018; 27 (12):1898-1902. DOI: 10.1177/0961203318797417 - 50.
Crow MK. Pathogenesis of systemic lupus erythematosus: Risks, mechanisms and therapeutic targets. Annals of the Rheumatic Diseases. 2023; 82 (8):999-1014. DOI: 10.1136/ard-2022-223741 - 51.
Kim JW, Kwok SK, Choe JY, et al. Recent advances in our understanding of the link between the intestinal microbiota and systemic lupus erythematosus. International Journal of Molecular Sciences. 2019; 20 (19):4871. DOI: 10.3390/ijms20194871 - 52.
Roda G, Chien NS, Kotze PG, et al. Crohn’s disease. Nature Reviews Disease Primers. 2020; 6 (1):22. DOI: 10.1038/s41572-020-0156-2 - 53.
Shouval DS, Rufo PA. The role of environmental factors in the pathogenesis of inflammatory bowel diseases: A review. JAMA Pediatrics. 2017; 171 (10):999-1005. DOI: 10.1001/jamapediatrics.2017.2571 - 54.
Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017; 390 (10114):2769-2778. DOI: 10.1016/S0140-6736(17)32448-0 - 55.
Ramos GP, Papadakis KA. Mechanisms of disease: Inflammatory bowel diseases. Mayo Clinic Proceedings. 2019; 94 (1):155-165. DOI: 10.1016/j.mayocp.2018.09.013 - 56.
Sanders ME, Merenstein DJ, Reid G, et al. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nature Reviews. Gastroenterology & Hepatology. 2019; 16 (10):605-616. DOI: 10.1038/s41575-019-0173-3 - 57.
Imhann F, Vich VA, Bonder MJ, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018; 67 (1):108-119. DOI: 10.1136/gutjnl-2016-312135 - 58.
Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biology. 2012; 13 (9):R79. DOI: 10.1186/gb-2012-13-9-r79 - 59.
Zhou L, Zhang M, Wang Y, et al. Faecalibacterium prausnitzii produces butyrate to maintain Th17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflammatory Bowel Diseases. 2018; 24 (9):1926-1940. DOI: 10.1093/ibd/izy182 - 60.
Takeshita K, Mizuno S, Mikami Y, et al. A single species of clostridium subcluster XIVa decreased in ulcerative colitis patients. Inflammatory Bowel Diseases. 2016; 22 (12):2802-2810. DOI: 10.1097/MIB.0000000000000972 - 61.
Birrenbach T, Bocker U. Inflammatory bowel disease and smoking: A review of epidemiology, pathophysiology, and therapeutic implications. Inflammatory Bowel Diseases. 2004; 10 (6):848-859. DOI: 10.1097/00054725-200411000-00019 - 62.
Odenwald MA, Turner JR. The intestinal epithelial barrier: A therapeutic target? Nature Reviews. Gastroenterology & Hepatology. 2017; 14 (1):9-21. DOI: 10.1038/nrgastro.2016.169 - 63.
Kudelka MR, Stowell SR, Cummings RD, et al. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nature Reviews. Gastroenterology & Hepatology. 2020; 17 (10):597-617. DOI: 10.1038/s41575-020-0331-7 - 64.
Gogokhia L, Buhrke K, Bell R, et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host & Microbe. 2019; 25 (2):285-299. DOI: 10.1016/j.chom.2019.01.008 - 65.
Van der Sluis M, De Koning BA, De Bruijn AC, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006; 131 (1):117-129. DOI: 10.1053/j.gastro.2006.04.020 - 66.
Saez A, Gomez-Bris R, Herrero-Fernandez B, et al. Innate lymphoid cells in intestinal homeostasis and inflammatory bowel disease. International Journal of Molecular Sciences. 2021; 22 (14):7618. DOI: 10.3390/ijms22147618 - 67.
Mitsialis V, Wall S, Liu P, et al. Single-cell analyses of colon and Blood reveal distinct immune cell signatures of ulcerative colitis and Crohn’s disease. Gastroenterology. 2020; 159 (2):591-608. DOI: 10.1053/j.gastro.2020.04.074 - 68.
Tindemans I, Joosse ME, Samsom JN. Dissecting the heterogeneity in T-cell mediated inflammation in IBD. Cell. 2020; 9 (1):110. DOI: 10.3390/cells9010110 - 69.
Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of Fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015; 149 (1):110-118. DOI: 10.1053/j.gastro.2015.03.045 - 70.
Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet. 2017; 389 (10075):1218-1228. DOI: 10.1016/S0140-6736(17)30182-4 - 71.
Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015; 149 (1):102-109. DOI: 10.1053/j.gastro.2015.04.001 - 72.
Vuyyuru SK, Kedia S, Kalaivani M, et al. Efficacy and safety of fecal transplantation versus targeted therapies in ulcerative colitis: Network meta-analysis. Future Microbiology. 2021; 16 :1215-1227. DOI: 10.2217/fmb-2020-0242 - 73.
Narula N, Kassam Z, Yuan Y, et al. Systematic review and meta-analysis: Fecal microbiota transplantation for treatment of active ulcerative colitis. Inflammatory Bowel Diseases. 2017; 23 (10):1702-1709. DOI: 10.1097/MIB.0000000000001228 - 74.
Cheng F, Huang Z, Wei W, et al. Fecal microbiota transplantation for Crohn’s disease: A systematic review and meta-analysis. Techniques in Coloproctology. 2021; 25 (5):495-504. DOI: 10.1007/s10151-020-02395-3 - 75.
Mayfield J. Diagnosis and classification of diabetes mellitus: New criteria. American Family Physician. 1998; 58 (6):1355-1362 1369-1370 - 76.
Han H, Li Y, Fang J, et al. Gut microbiota and type 1 diabetes. International Journal of Molecular Sciences. 2018; 19 (4):995. DOI: 10.3390/ijms19040995 - 77.
Zheng P, Li Z, Zhou Z. Gut microbiome in type 1 diabetes: A comprehensive review. Diabetes/Metabolism Research and Reviews. 2018; 34 (7):e3043. DOI: 10.1002/dmrr.3043 - 78.
Stojanovic I, Saksida T, Miljkovic D, et al. Modulation of intestinal ILC3 for the treatment of type 1 diabetes. Frontiers in Immunology. 2021; 12 :653560. DOI: 10.3389/fimmu.2021.653560 - 79.
Gulden E, Chao C, Tai N, et al. TRIF deficiency protects non-obese diabetic mice from type 1 diabetes by modulating the gut microbiota and dendritic cells. Journal of Autoimmunity. 2018; 93 :57-65. DOI: 10.1016/j.jaut.2018.06.003 - 80.
Niinisto S, Takkinen HM, Erlund I, et al. Fatty acid status in infancy is associated with the risk of type 1 diabetes-associated autoimmunity. Diabetologia. 2017; 60 (7):1223-1233. DOI: 10.1007/s00125-017-4280-9 - 81.
Tai N, Peng J, Liu F, et al. Microbial antigen mimics activate diabetogenic CD8 T cells in NOD mice. The Journal of Experimental Medicine. 2016; 213 (10):2129-2146. DOI: 10.1084/jem.20160526 - 82.
Burrows MP, Volchkov P, Kobayashi KS, et al. Microbiota regulates type 1 diabetes through toll-like receptors. Proceedings of the National Academy of Sciences of the United States of America. 2015; 112 (32):9973-9977. DOI: 10.1073/pnas.1508740112 - 83.
LeBlanc JG, Chain F, Martin R, et al. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microbial Cell Factories. 2017; 16 (1):79. DOI: 10.1186/s12934-017-0691-z - 84.
Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016; 535 (7610):75-84. DOI: 10.1038/nature18848 - 85.
Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009; 139 (3):485-498. DOI: 10.1016/j.cell.2009.09.033 - 86.
Gazali AM, Schroderus AM, Nanto-Salonen K, et al. Mucosal-associated invariant T cell alterations during the development of human type 1 diabetes. Diabetologia. 2020; 63 (11):2396-2409. DOI: 10.1007/s00125-020-05257-7 - 87.
Wong EB, Ndung'U T, Kasprowicz VO. The role of mucosal-associated invariant T cells in infectious diseases. Immunology. 2017; 150 (1):45-54. DOI: 110.1111/imm.12673 - 88.
Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: Immunobiology and pathology. Annual Review of Immunology. 2015; 33 :747-785. DOI: 10.1146/annurev-immunol-032414-112123 - 89.
Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nature Reviews. Immunology. 2014; 14 (12):783-795. DOI: 10.1038/nri3766 - 90.
Maxwell JR, Zhang Y, Brown WA, et al. Differential roles for Interleukin-23 and Interleukin-17 in intestinal Immunoregulation. Immunity. 2015; 43 (4):739-750. DOI: 10.1016/j.immuni.2015.08.019 - 91.
Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015; 43 (4):727-738. DOI: 10.1016/j.immuni.2015.09.003 - 92.
Liang B, Wu C, Wang C, et al. New insights into bacterial mechanisms and potential intestinal epithelial cell therapeutic targets of inflammatory bowel disease. Frontiers in Microbiology. 2022; 13 :1065608. DOI: 10.3389/fmicb.2022.1065608 - 93.
Otte ML, Lama TR, Papapanagiotou J, et al. Mucosal healing and inflammatory bowel disease: Therapeutic implications and new targets. World Journal of Gastroenterology. 2023; 29 (7):1157-1172. DOI: 10.3748/wjg.v29.i7.1157 - 94.
Mougiakakos D, Kronke G, Volkl S, et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. The New England Journal of Medicine. 2021; 385 (6):567-569. DOI: 10.1056/NEJMc2107725 - 95.
Xiao L, Van'T LB, van de Worp W, et al. Early-life nutritional factors and mucosal immunity in the development of autoimmune diabetes. Frontiers in Immunology. 2017; 8 :1219. DOI: 10.3389/fimmu.2017.01219