Predicted antigenic determinants for T cells (HLA-B13 decamers) according to database SYFPEITHI.
Abstract
In the study of “the herd immunity”, it was found that antigenic “late” proteins L1 of human papillomavirus of types 6,16,18,31 and 45, “early” proteins E2, E6 and E7 induced the generation of interferon, CD4/CD8 T lymphocytes and T cell receptors, as well as apoptotic enzymes: granzyme B, perforin and granulysin in mice peripheric mononuclear blood cells and in splenocytes (according to Elispot). Cancer HeLa cells provoked tumour formation in mice testis and in intact lungs in a month after injection and in isolated lungs after 1–2 days of inoculation. “Early” protein E2, L-amino acid oxidase and D-amino acid oxidase blocked the growth of HeLa cells in vitro, working as an effector. There was the activation of the generation of interferon, immunogenic T lymphocytes as well as apoptotic enzymes: granzyme B, perforin and granulysin in blood, spleen and lung T lymphocytes in tumours of isolated lungs mice treated with HeLa cells. Even when anti-PD-L1 antibody (“checkpoint” control receptor for cancer blocking) was added to isolated tumorigenic mice lung, regardless of the presence of HeLa cells, there was the induction of the immunogenicity. The testing of immunogenic and oncolytic activities of antigens via isolated lung tumour formation lasted 5–7 days including Elispot and HeLa inoculation and provided rapid analysis of immunogenic effector activity and tumour suppressors.
Keywords
- human papillomavirus (HPV)
- antigenic “early” and “late” proteins of HPV (6
- 16
- 18
- 31 and 45 types)
- induction of the generation of interferon
- CD4/CD8 T lymphocytes
- T cell receptor
- regression of mice testis and lung tumours by HPV16 E2
- activation of apoptotic enzymes: Granzyme B
- perforin and granulysin
- immunogenic and oncolytic effector activities of L-amino acid oxidase and D-amino acid oxidase
1. Introduction
Immune surveillance of cancer is very necessary for host surviving in order to escape carcinogenesis [1]. According to data GLOBOCAN (WHO Agency) in 2022 year, there were registered as much as 1,918,030 new cancer cases and 609,360 cancer deaths that means 350–400 deaths cases per day [2].
Cancer in most cases is a comorbidity and mortality incidence together with HIV, hepatitis B and papillomavirus (HPV) diseases because of weakening of the immune system [3, 4]. Even in every cancer overgrowth, there were usually found papillomaviruses.
Therefore there is an urgent need to develop strategy provided common protection from infections and toxins for therapeutic vaccine, that can fully eliminate malignant cells.
In adult humans, the number of T lymphocytes is close to the quantity of 1011–15 mostly representing naive cells, which means they are uncharged by any epitopes [5, 6]. These naive T cells resident 40% of the spleen, in peripheral blood up to 50%, and the other of all internal organs: lungs, liver, lymphatic organs etc., have their own depot of T cells as well naive to be ready for activating by external stimuli. The life span of different T lymphocytes can be: for effector natural killer cells of the order 8–10 days, for most peripheral T cells can remain in a resting state for a long period (months in rodents and years in humans) [7, 8].
The huge amount of T naive cells allows us to suspect that their turnover is not so long, and nevertheless, the resting (even still huge) amount of T cells needs to be continuously activated by different effectors in order to support the immune system successfully [9].
The aim of a therapeutic vaccine against cancerogenic HPV is to induce in vivo virus-specific T-cell response against established HPV infections and lesions. At the same time, be sure that vaccine-induced T cells can reach the tumour site and perform their functions without limitation.
A range of approaches were undertaken for the creation of therapeutic vaccines against HPVs: increasing CD4/CD8 T cell response, DNA, RNA, attenuated viral and bacterial, peptide-based, protein-based or on the basis of dendritic cells. But no vaccines were licenced for therapeutic use [10].
The most attention attracted the “early” oncogenes: E6 targeting oncosupressor p53 and E7 interacting with pRB and reducing oncogenic strength. But no significant results were achieved [10]. The therapeutic vaccine against recurrent respiratory papillomatoses on the base vaccinia virus MVA with bovine “early” protein E2 BPV completely regressed laryngeal papillomatosis. The immune response was assessed by the increase of CD4/CD8 T lymphocytes [11].
In order to develop the oral therapeutic vaccine, the aim of our work was to elaborate on the rapid screening system of antigens in order to evaluate their immunogenic and oncolytic specificities and activities. For this purpose, antigenic proteins of papillomaviruses of high-risk oncogenes types of HPV16, 18, 31, 45 and unrelated anogenital type of HPV6 of major coat protein L1 of the “late” expression of the viruses (Figure 1) were employed. The regulatory protein of “early” expression of HPV16 E2, well known as tumour supersupressor, was recruited to study its immunogenic and oncolytic activities for the perspectives of the development of a therapeutic vaccine against cancer.
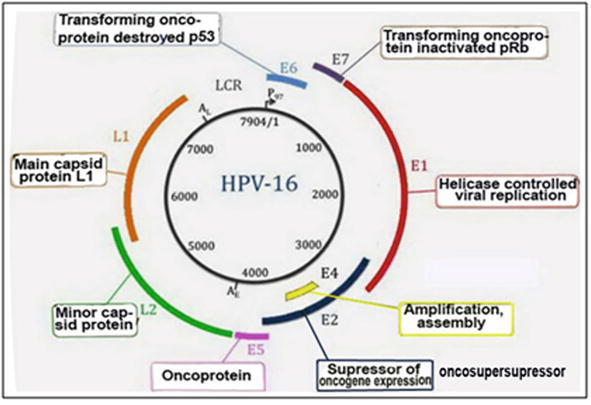
Figure 1.
Physical map of high-risk oncogenic virus HPV16.
The fast-growing tumours perhaps revealed a rapid protein/amino acid turnover, so the L-amino acid oxidase (LAAO) and D-amino acid oxidase (DAAO) were chosen to study their participation in tumour regression.
2. Methodology
2.1 The genetic constructs and synthetic capacity of the plant virus expression system on the basis of tomato fruit
To synthesise antigenic proteins of HPV6 L1, HPV16, 18, 31 and 45 L1, HPV16 E2, our own author genetic constructs were designed in which RNA-dependent RNA-polymerase (RdRP from Cucumber mosaic virus) played the pivotal role [12]. According to our design, a company Genscript (USA), synthesised eight genetic constructs for the development of prophylactic and therapeutic vaccines against HPV viruses for the production of target proteins in plant viral expression system on the basis of tomato fruit (Figure 2).
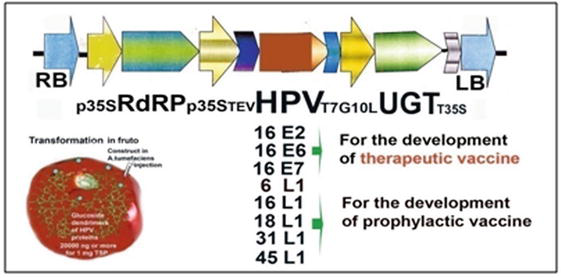
Figure 2.
The plant viral expression system for the production of target antigenic HPV proteins on the basis of tomato fruit.
2.2 Qualitative and quantitative analyses
SDS electrophoresis has been done in Phastsystem apparatus with appropriate buffer strips and ready-to-use plates with PAAG gradient of 8–15% (Amersham, UK). The programme attached to the device was №3 for protein separation [12, 13, 14].
The quantities of antigenic proteins synthesised in tomato were estimated by ELISA with appropriate target standard of commercial papillomavirus proteins from companies Santa Cruz Biotech. Inc. and Genway Biotechnology Inc. (USA) [12, 13]. Commercial primary and secondary antibodies of the same companies were used in ELISA.
For Elispot analyses, peripheral mononuclear blood cells (PMBC), splenocytes and T lymphocytes from mice lungs were used. For the isolation of T lymphocytes, fresh or stored at
These preparations were used in Elispot analyses with antibodies of a company Abcam recruiting HPV16 E2, LAAO or DAAO as activators (effectors). To study the activation of immune system, Elispot analysis was provided with antibodies from the company Abcam (UK) as follows: murine IFNγ ELISPOT KIT [AB64029], rabbit monoclonal [EPR1108] to interferon-gamma (AB133566), Anti-T-Cell Receptor antibody (JOVI.1) (AB5465) mouse monoclonal, rabbit monoclonal Anti-CD4 antibody [EPR19514] (AB183685), rabbit monoclonal Anti-CD8 alpha (SP16) antibody [EPR21769] (AB217344) [14, 15, 16, 17], rabbit monoclonal antibodies for enzymes of apoptosis: rabbit polyclonal Anti-Granzyme B (AB53097), rat monoclonal Anti-Perforin antibody [CB5.4] (AB16074), Anti-Human Granulysin (AB213787) monoclonal. Second antibody were goat immunoglobulins to mice conjugated with alkaline phosphatase and substrates BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate/nitrotetrazolium blue (Sigma, USA).Anti-PD-1 Antibody Clone G4 MABC1132-100UG, monoclonal, from hamster (Armenian) Sigma-Aldrich, USA) and Anti-PDL1M1/CLP36 antibody [EPR7186] ab129015 rabbit monoclonal were used in the experiments to control “check-point” receptors in mice lung tumours.
2.3 The description of experiments with mice
Mice were grown in vivarium with standard conditions. All necessary activities for their maintenance were completed. For experiments, baby mice were selected and grown up in the same cage in order to escape differences in keeping.
HeLa cells were purchased from the company “Biolot” (Saint Petersburg, Russia Federation) and were grown in Corning flasks in DMEM with 10% of bovine fetal serum. Before experiments, HeLa cells were kept at −62
Mice orally vaccinated at the age of 6 months with 500 mg of HPV16 E2 from tomato vaccine material three times with the interval of 1 month. Collected samples of peripheral blood were stored at -62
In some experiments, injected mice were orally vaccinated after one month, and after 1 month of the last vaccination, they were used for blood sampling and the isolation of lungs and spleen.
For histological analyses and to study the cell state in lung tumours that appeared after the inoculation of lungs with cancer HeLa cells and after vaccination, the microtome slices of these lung tumours were prepared via classical section waxing techniques and staining with haematoxylin by Carazzi. A light microscope with magnification x 360–900 was equipped with a video camera with programme C310 NG Levenhuk (USA) with a resolution of 2048–1536 pc.
3. Results. The study of immunogenic activities of antigenic prophylactic and therapeutic proteins
3.1 The possibility of using prophylacic vaccines as therapeutics
Our plant viral expression system, according to design, produced as much as 25–30 μg of the corresponding antigenic protein per 1 mg of total soluble protein (TSP) [12]. Only one appropriate band was found after electrophoresis without any other impurities in the field that allowed the use of crude buffer extracts of transgenic tomato fruit to escape any multistep and cost purification (Figure 3) [12, 13, 14].
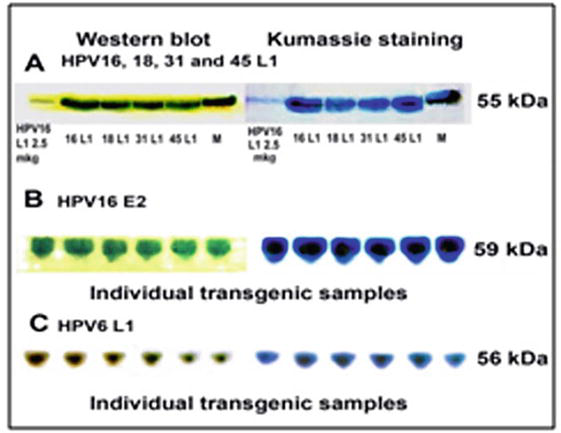
Figure 3.
The electrophoresis and western blot of crude buffer extracts with antigenic proteins: A - HPV16, 18, 31, 45 L1; B - HPV16 E2; C - HPV6 L1 synthesised in plant viral expression system on the basis of tomato fruit. Standard - 2.5 μg of HPV16 L1 (Santa Cruz biotech., USA).
All these antigenic proteins synthesised in tomato fruit were used for the induction of antibody synthesis in peripheral blood of mice (Figure 4).
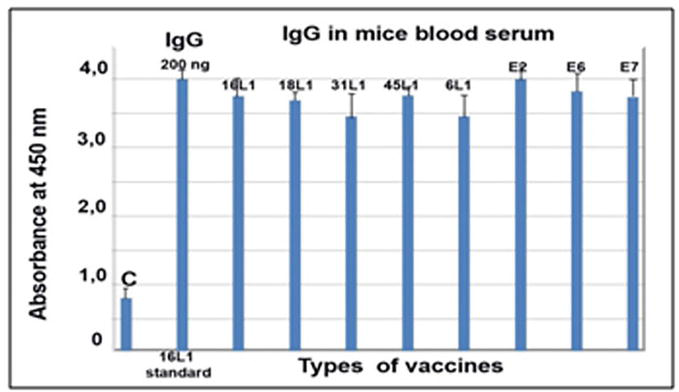
Figure 4.
Antibodies to different types of antigenic proteins of HPV developed in mice blood serum after oral vaccination of mice with appropriate oral vaccines. 200 ng HPV16 L1 is a standard from company Santa Cruz biotech (USA). C-serum from control unvaccinated mice.
Mice blood serum samples are the excellent model to study the antibody development and the immune system activation upon oral vaccination. Antibodies raised in mice in our experiments (Figure 4) have strong avidity, very high titre and broad specificity to different types of antigens of HPV L1. According to bioinformatic analyses (gathered from database SYFPEITHI), there are common epitops (decamers) in all proteins sequences of HPV L1 (means related to one family of 16, 18, 31, 45 L1 types and unrelated from noncarcinogenous anogenital 6 L1 type) but not to HPV16 E2 or LAAO or DAAO of protein sequences (Table 1).
| Type of HPV | Position | Peptide |
|---|---|---|
| HPV16 L1 | ||
| 304 | *AQIFNKPYWL | |
| 372 | ||
| HPV18 L1 | 181 | SQGDCPPLEL |
| 304 | ||
| HPV31 L1 | ||
| 69 | LQYRVFRVRL | |
| 373 | LQFIFQLCKI | |
| HPV45 L1 | ||
| 307 | SQLFNKPYWL | |
| 464 | DQYPLGRKFL | |
| HPV6 L1 | 86 | SLFDPTTQRL |
| 300 | AQLFNKPYWL | |
| 101 | GLEVGRGQPL | |
| HPV16 E2 | 199 79 350 | GQVILCPTSV LQAIELQPTL WQRDQFLSQV |
| L-amino acid oxidase [from Crotalus adamanteus] LAAO | 271 426 171 | VQVHFNARVI IQTFCHPSMI QLYVESLRKV |
| D-amino acid oxidase [from pig kidney] DAAO | 203 97 296 | LLQPGRGQII GLTPVSGYNL PQVRLEREQL |
Table 1.
Similar epitops (decamers) are coloured in blue and yellow.
Antigenic protein from unrelated noncarcinogenous anogenital type HPV6 L1 was able to be highly active in the induction of the synthesis of interferon (INF), CD4/CD8 ligands of T lymphocytes from peripheral mononuclear blood cells (PMBC) of mice orally vaccinated with tomato vaccine material transgenic with HPV16, 18, 31 and 45 L1 types (Figure 5) (Table A1) [14].
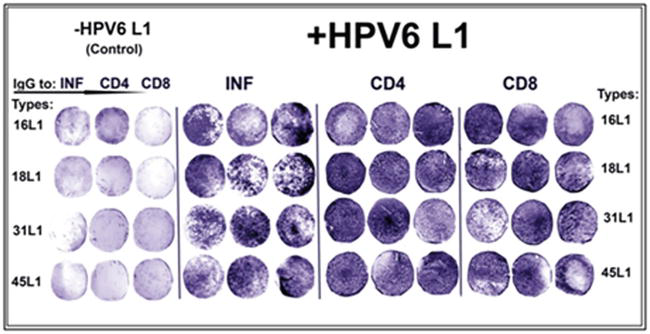
Figure 5.
The induction of the synthesis of interferon (INF), CD4 and CD8 T lymphocytes in peripheral mononuclear blood cells from mice orally vaccinated with vaccines of antigenic HPV16, 18, 31 and 45 L1 by another antigenic protein of phylogenetically unrelated type of papillomavirus HPV6 L1. Control - the inoculation of T lymphocytes from blood of corresponding variants of vaccinated mice was without HPV6 L1.
(Here and further the numbers of coloured immunospots were presented in the Tables in Supplement).
Due to the fact that antigenic coat protein L1 of types HPV16, 18, 31 and 45 usually used for the development of prophylactic vaccines like Gardasil or Cervarics but not for the therapeutical vaccines, was able to induce immune response, it might be consumed that there was a block step on the way lying of above mentioned antigenic proteins L1 to the direction provided the regression of tumours.
Conversely, there is no need to create multivalented prophylactic vaccines like 9-Gardasil if a great immune response is possibly provided by few antigenic proteins of high immunogenic activity.
Nevertheless, it cannot be excluded that the therapeutical potential of prophylactic vaccines should be investigated and improved using several approaches.
3.2 The experimental induction of carcinogenesis by HeLa cells injected in mice
To evoke carcinogenesis, the injection of 100 μl of suspension of cancer HeLa cells was done into the hip muscle of male or female mice, and after 1 month of the inoculation, different tumours were observed outside and inside of mice bodies (Figure 6).
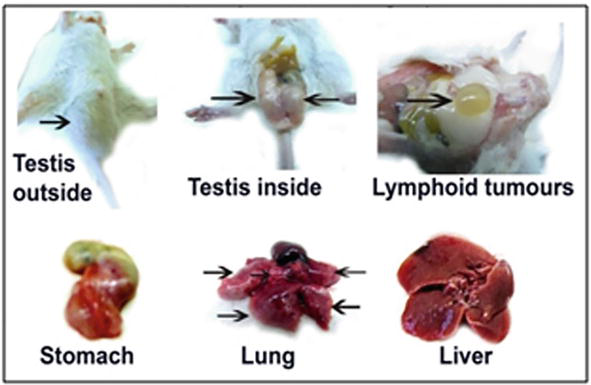
Figure 6.
Tumour formation in mice bodies after 1 month of the injection of cancer HeLa cells. Arrows indicate tumours.
To induce the regression of the testis tumours, the antigenic oncosupersupressive protein HPV16 E2 was employed. As can be observed in Figure 7, testis tumours appeared 1 month after the injection of mice with cancer cells HeLa. With these tumours male mice stayed still alive and survived during another half a year. Upon ELISA and Elispot analyses, there was registered a significant increase in the content of interferon (INF) and in the amount of generations of CD4/CD8 T lymphocytes in PMBC and in splenocytes (Figure 8). After the vaccination with HPV16 E2 (500 mg per mouse), in 2 days, testis tumours became smaller and later disappeared after 1–2 months [15].
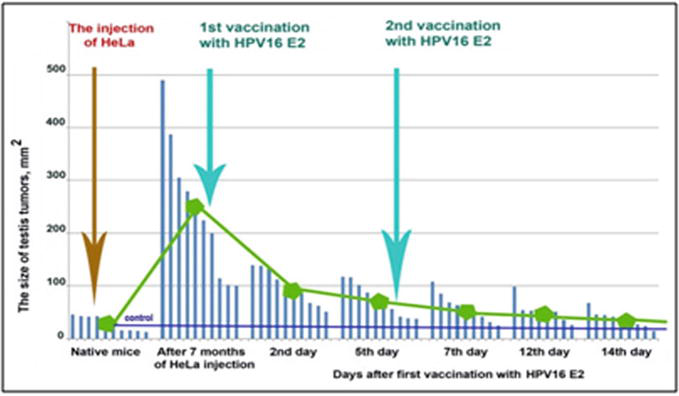
Figure 7.
The regression of testis tumours in male mice after the vaccination with HPV16 E2. N = 10 mice.
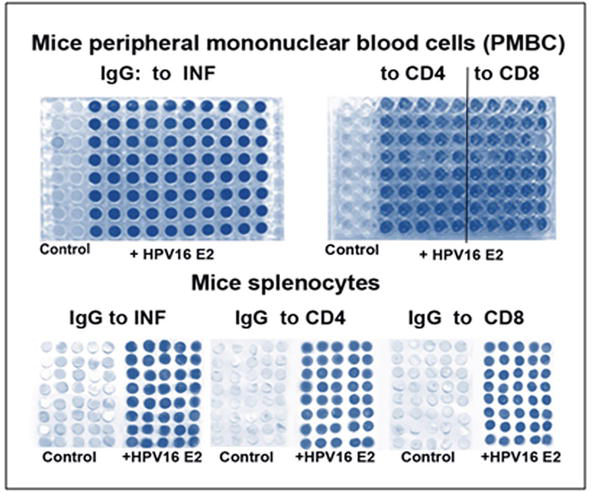
Figure 8.
The Elispot (left and bottom) and ELISA (upper right) analyses the increase of the synthesis of interferon (INF) and enlarging of generations of CD4/CD8 T lymphocytes both in PMBC and splenocytes. Control - without HPV16 E2.
The content of newly synthesised INF in PMBC and splenocytes was close to 62 ng per well of the microplate in ELISA analysis. In addition, the contents of CD4/CD8 T lymphocytes increased after vaccination as well were significantly high (Figure 9).
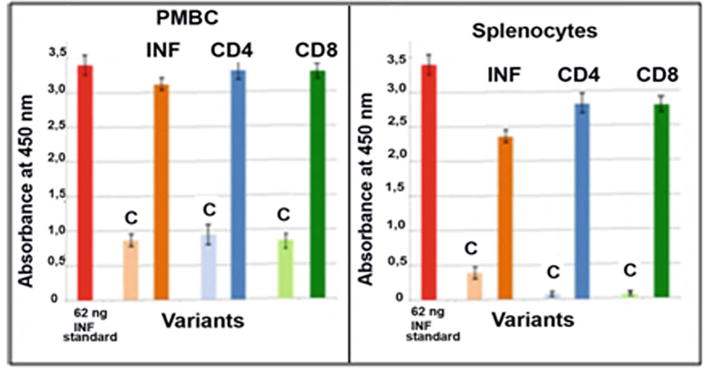
Figure 9.
ELISA analysis of the contents of INF, CD4/CD8 T lymphocytes after in PMBC and splenocytes of mice orally vaccinated with tomato vaccine material with HPV16 E2. C-control [
During the investigation of internal tumours that appeared in mice bodies after the injection with cancer HeLa cells, it was found that mice lungs were very sensitive and began to proliferate in the next few days with tumours formation of different sizes and types (Figure 10).
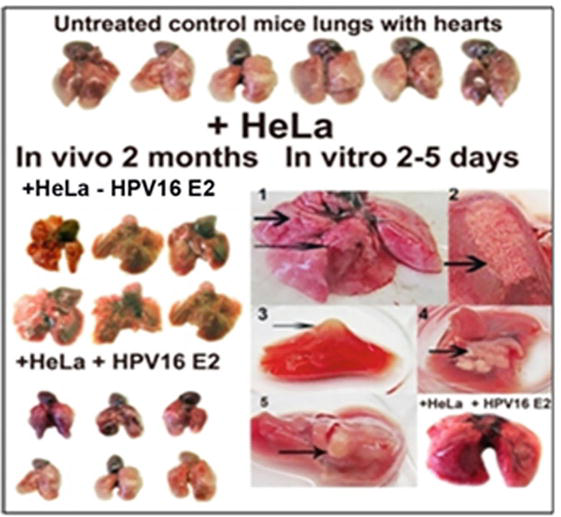
Figure 10.
The sensitivity of mice lungs to the inoculation with HeLa cells. №№ 1–5 - tumours of different types observed on the surface of isolated mice lungs after 2–5 days of inoculation with HeLa cells. The oral vaccination with HPV16 E2 of mice previously incubated with HeLa cells abolished the tumour formation in isolated mice lung (to the right bottom), variant +HeLa+HPV16 E2.
The effect of the injection of HeLa cells on the morphology of lungs of mice (both male and female) and the regression of the morphology changing after oral vaccination with vaccine material of tomato fruit with “early” protein HPV16 E2 could be seen in Figure 10. Control variant (+HeLa-E2) of mice lungs without treatment – mice lungs after 2 months after the injection with HeLa cells and vaccination with HPV16 E2. Variant (+HeLa+E2) - mice lungs after 2 months after the injection with HeLa cells and then after 2 months of double vaccination with HPV16 E2 with the interval of 1 month. Lungs of mice only vaccinated with HPV16 E2 were without any changes (not shown in Figure 10). Lungs were presented together with hearts because of easier manipulation with them by holding hearts with tweezers.
When it was found that the one-time incubation of isolated mice lungs with HeLa cells and HPV16 E2 cancelled the formation of the tumours in isolated mice lungs and oral vaccination with HPV16 E2 abolished the tumour formation and resulted in tumour regression, it was decided to analyse the direct action of HPV16 E2, E6, E7, LAAO and DAAO on growing cancer HeLa cells. The action was estimated by using the staining with trypan blue dye-coloured dead cells because of the destruction of cell membranes.
As can be seen from Figure 11, antigenic proteins HPV16 E6 and HPV16 E7 did not influence HeLa cells (№ 2 and № 3), but HPV16 E2, LAAO and DAAO killed HeLa cells because the trypan blue dye showed dark blue colour and destroyed cell structures (cell debris) appeared.
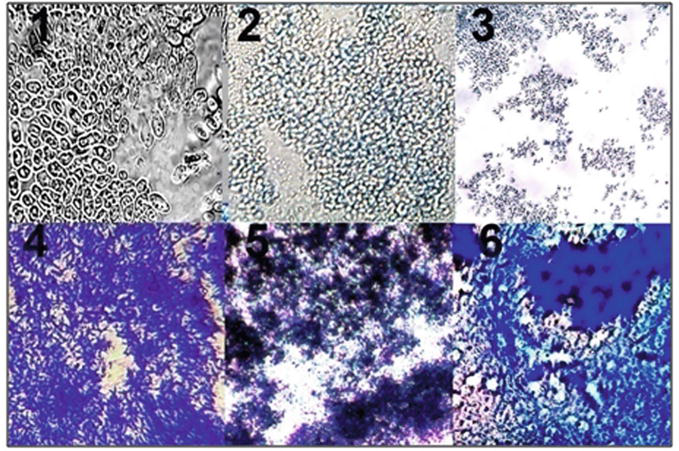
Figure 11.
The effect of the “early” proteins HPV16 E2, E6, E7, LAAO and DAAO on cancer HeLa cells. 1 - cells of fresh-grown suspension of HeLa cells, 2 - HeLa cells in the presence of HPV16 E6 and trypan blue (TB), 3 - the absence of staining of HeLa cells with TB in the presence of HPV16 E7, 4 - the full destruction of HeLa cells and staining with TB after the addition of the HPV16 E2, 5 - the destruction of HeLa cells and of staining with TB in the presence of LAAO, 6 - the destruction of HeLa cells and staining with TB in the presence of DAAO.
Tumour overgrowths cones and waves of cell proliferation with dark-coloured hyperchromic nuclei that could be seen in Figure 12 were analogs of small-round-cell lung sarcoma with hyperchromic stained nuclei.
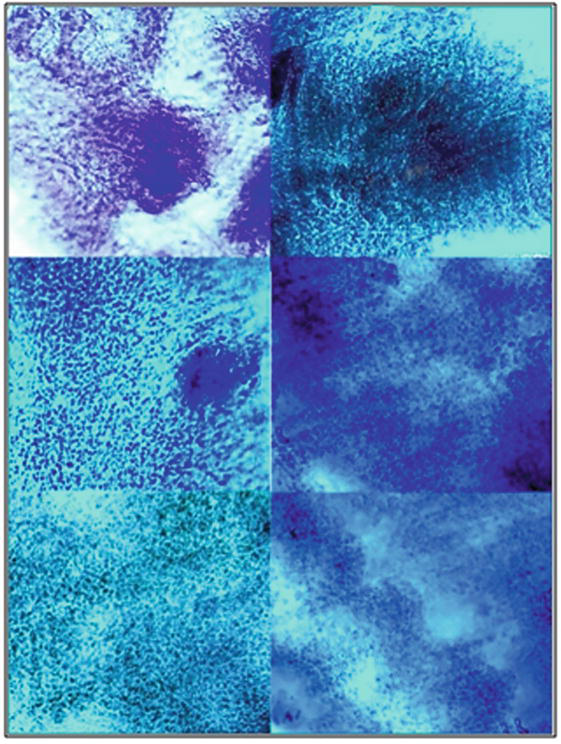
Figure 12.
Patterns of microtome slices of mice lungs after 5 days of inoculation with cancer HeLa cells (variant +HeLa-E2) without vaccination. Slices were stained with haematoxylin by Carazzi.
In Figure 13, it could be seen patterns of normal lung tissues: flattened cells of peripheral lung parenchima with faintly coloured nuclei, patterns of smooth muscle elongated cells and their transverse sections with pale coloured nuclei of myocytes, patterns of bronchioles elements, loose connective tissue cells with rare hyperchromic nuclei. There were no huge proliferative areas of dividing cells with hyperchromic stained nuclei into slices made of normal lung tissues without HeLa. In order to study immunogenic and oncolytic activities of HPV16 E2, peripheral mononuclear blood cells (PMBC) and splenocytes orally vaccinated with the HPV16 E2 tomato vaccine were isolated according to methods [17, 18].
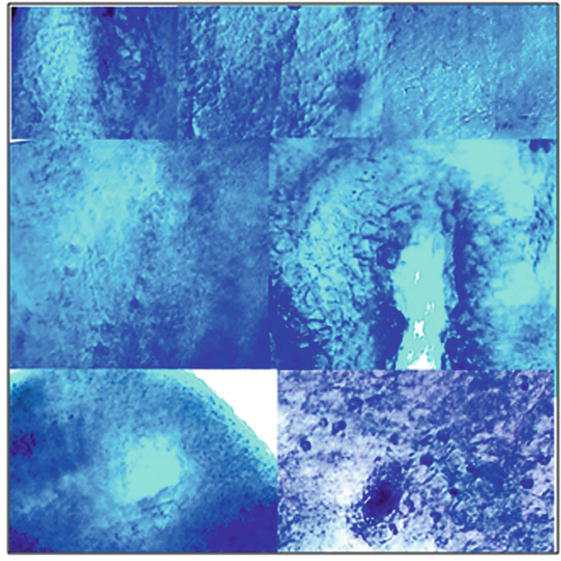
Figure 13.
Patterns of microtome slices of mice lungs after 5 days of the inoculation with cancer HeLa cells in the presence of vaccine material of tomato fruit transgenic with HPV16 E2 (variant +HeLa+E2). Slices were stained with haematoxylin by Carazzi.
As it was seen from Figures 14 and 15, (Table A2) the oral vaccination mice with vaccine material containing antigenic protein HPV16 E2 highly increased the synthesis of γ-interferon, accelerated the generation of CD4/CD8 T lymphocytes and activated apoptotic enzymes: granzyme B, perforin and granulysin.
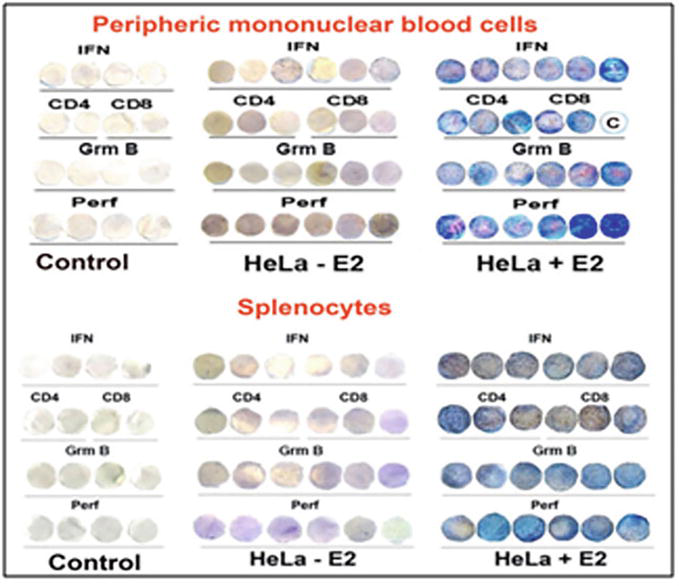
Figure 14.
Elispot analysis of the activation of the synthesis of interferon (INF), CD4 and CD8 T lymphocytes, apoptotic enzymes: Granzyme B (GrmB) and perforin (perf) in peripheral mononuclear blood cells and in splenocytes, preliminary injected with cancer HeLa cells and then vaccinated with vaccine material of tomato fruit with transgenic HPV16 E2. Letter “C” means control [
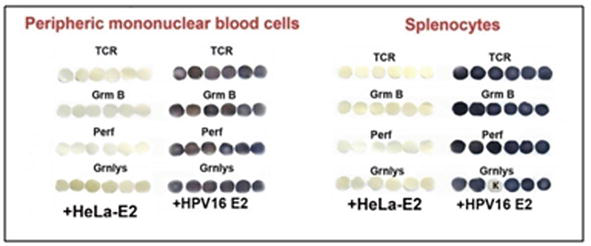
Figure 15.
Elispot analysis of the activation of the synthesis of T cell receptors (TCR) and apoptotic enzymes: Granzyme B (GrmB), perforin (perf) and granulysin (Grnlys) in peripheral mononuclearblood cells and in splenocytes of mice injected with cancer HeLa cells and thеn vaccinated with vaccine material of tomato fruit transgenic with HPV16 E2. Control (+HeLa-E2) - mice injected with cancer HeLa cells without vaccination. Letter “K” in the lower row to the right means empty membrane disk without adding of splenocytes.
In attempts to decipher the oncolytic effect of HPV16 E2, the Elispot analysis with antibodies to protein inhibitors of check-point control of carcinogenesis was undertaken with antibodies to the receptor on T lymphocytes PD-1 and to cancer receptor PD-L1 (Figure 16) (Tables A3 and A4).
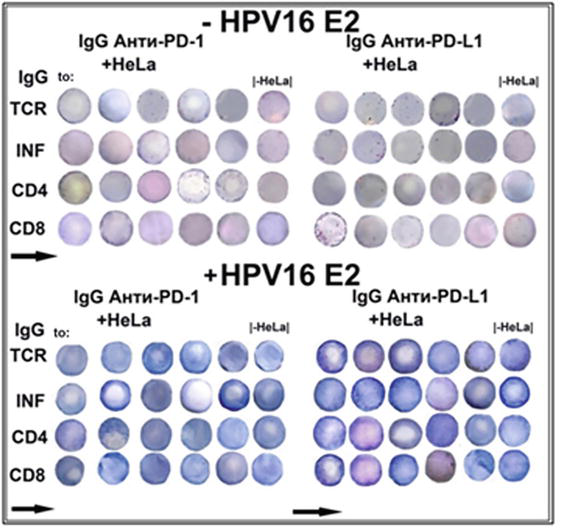
Figure 16.
Elispot amalyses of the activation of immune response in colonies of T lymphocytes isolated from mice lungs after the inoculation in the suspension of HeLa cells in the presence of antibodies to check-points PD-1 and PD-L1 evoked by vaccine material of tomato fruit transgenic with HPV16 E2. (see details in the text).
To obtain these results, fresh isolated mice lungs (12 mice at the age of 6 months and masses of 35–40 g) were placed in Corning flasks with DMEM nutritive medium in which cancer HeLa cells were grown. At this time, the solution of antibodies to PD-1 and PD-L1 (each 10 μl) was put in the flasks. Flasks were left for 2 days until tumour overgrows became visible on the surface of the lungs. Then lungs were minced with a syringe needle in 1 ml of DMEM, and homogenates were placed in a centrifuge tube and floated at 700 g for 7 minutes at 4°C as described in [16, 17, 18]. Aliquots of 20 μl were added to wells of Corning microplate (25 wells) on nitrocellulose disks (12 mm in diameter) with 300 μl of DMEM. 100 μg of the water solution (100 μl) of HPV16 E2 was placed, and the incubation was continued for 5 days. After that, the procedure was as follows [17, 18]. It was found that when the cancer checkpoint was inhibited with antibody to PD-L1, HPV16 E2 highly activated the formation of T cell receptors (TCR) and increased the activity of apoptotic enzymes: granzyme B, perforine and granulysin.
As a matter of fact, as a whole, the immune system of mice lungs appeared to be less active up to 100 times (at least) than it was in the spleen or in PMBC. Gentle mice lung cells might be unable to resist the invasion of HeLa cells and not survive. Nevertheless, the addition of HPV16 E2 effectively increased the synthesis of interferon (INF), T cell receptors (TCR) and accelerated the generation of CD/CD8 T lymphocytes, even in lung T lymphocytes damaged with HeLa cells.
LAAO and DAAO appeared to have immunogenic activities (Figure 17) (Tables A5 and A6) in blood and spleen isolated from mice.
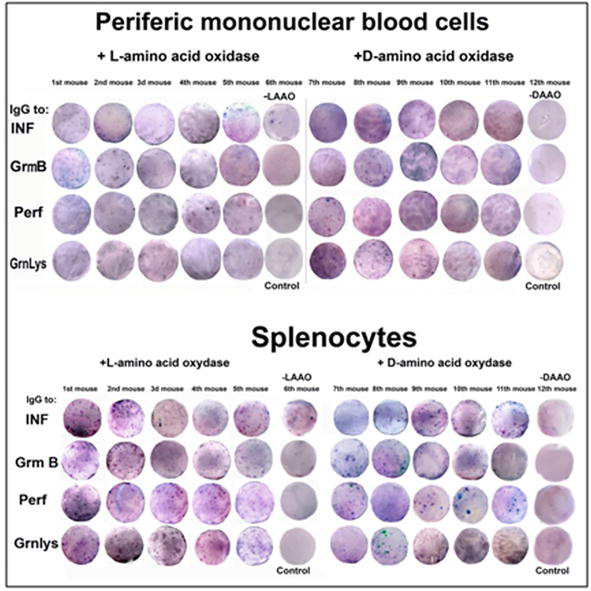
Figure 17.
Elispot analysis of immunogenic activity of LAAO and DAAO in PMBC and splenocytes of untreated mice.
To appreciate the immunogenic and oncolytic effectivities of L-amino acid and D-amino acid oxidases (LAAO and DAAO), the experimental design was employed as follows: fresh isolated mice lungs (at the age of 6 months with masses 35–40 g) was placed in Corning flasks with the suspension of growing cancer HeLa cells per 1 day. Tumours of overgrowths appeared on the surface of the lungs the next day after placing of lungs into flasks (Figure 18). To flasks, 10 mg of LAAO or DAAO in DMEM solution were added separately. On the next day after oxidase addition, the tumours became smaller and later disappeared on 5th–6th days of inoculation, revealing a smooth surface. These lungs were used in Elispot analysis to evaluate the immunogenic activities of LAAO and DAAO (Figure 19).
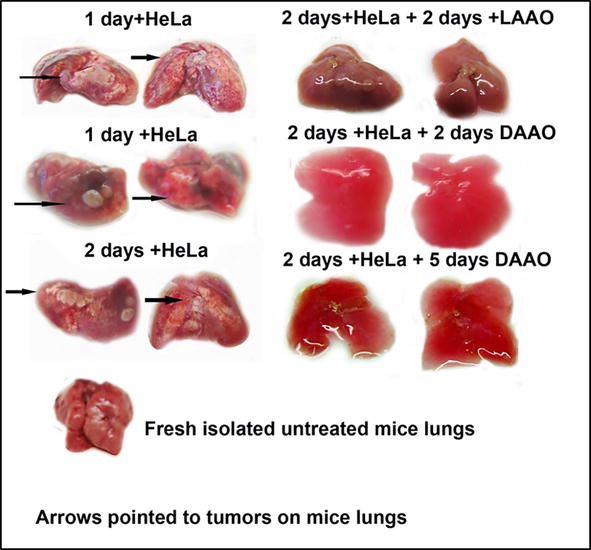
Figure 18.
The effect of LAAO and DAAO on tumours of mice lungs evoked by cancer HeLa cells.
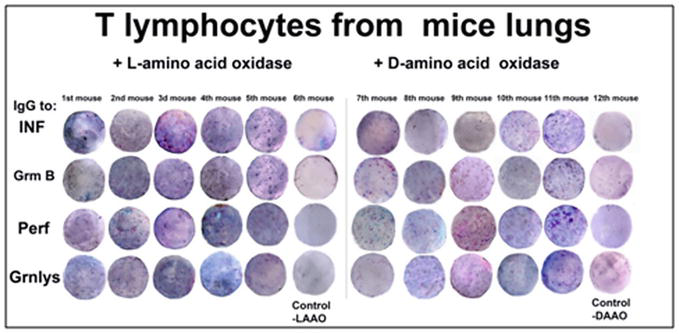
Figure 19.
Elispot analysis of LAAO and DAAO immunogenic activity in T lymphocytes isolated from mice lungs inoculated with cancer HeLa cells.
In order to understand what happened with a large area of lungs with tumour cells having hyperchromic nuclei, the microtome slices were prepared (Figure 20).
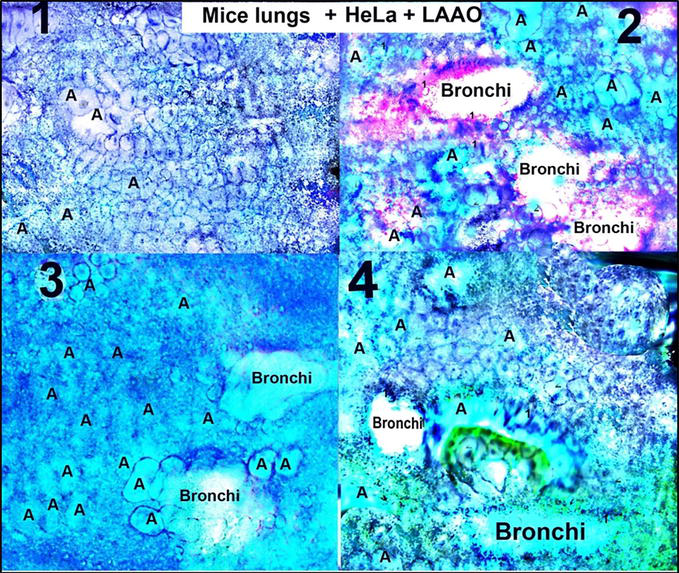
Figure 20.
Microtomic slices prepared from lungs treated at first with cancer HeLa cells and after inoculated with LAAO (from
There were not found widespread areas of round cancer cells having hyperchromic nuclei in microtomic slices prepared from lungs at first inoculated with HeLa and after being treated with LAAO. There were presented cells, and tissues resembled patterns of smooth muscle elongated cells with pale coloured nuclei of myocytes, flattened cells of peripheral lung parenchyma with faintly coloured nuclei, and loose connective tissue cells with rare hyperchromic nuclei. Alveolocytes (A) and bronchioles (bronchi) clearly could be seen (Figure 20).
From the results presented in Figures 18–20, (Table A7) it might be concluded that L-amino acid oxidase (LAAO) and D-amino acid oxidase (DAAO) both were active as activators (effectors) of the immune response in peripheral mononuclear blood cells and in splenocytes. But their actions were more pronounced on the mice lung T lymphocytes treated with cancer HeLa cells. This is the first evidence of the activity of LAAO and DAAO on the immune system and especially on the immune system of mice lungs after the invasion of cancer HeLa cells. This observation is in good correlation with the results of Table 1 in that it was shown the predicted antigenic determinants for T cells (HLA-B13 decamers) determined with the database SYFPEITHI. Both LAAO and DAAO have potent epitopes but without any similarity to other antigenic proteins used in this study.
4. Discussion
As a matter of fact, lungs are chronically exposed to an array of cellular and airborne stressors, including aerosols, infectious agents, allergens, pollutants, pneumotoxic chemicals, medications and other biomedical injuries. To overcome these biological and environmental stresses, the lungs must make lower the immune response as a whole [19]. Pulmonary lipoproteins have been shown to modulate the inflammatory response to microbial components. These surfactants were able to inhibit macrophage proinflammatory cytokine response and reduced inflammatory mediator production by blocking the toll-like receptor 2 (TLR2) pathways, thus repressing lung inflammation caused by internal stresses [20]. For example, pulmonary surfactants are complex and highly surface-active materials that are found in the fluid lining of the alveolar surface and play an important role in stabilising breathing, pulmonary homeostasis and mucosal immunity [20]. Human surfactant protein D (SP-D) facilitates SARS-Cov-2 pseudotype binding, and entry with pattern recognition receptors having the crucial immune function in detecting, and clearing pulmonary pathogens and downregulates spike protein-induced inflammation [21]. Recombinant fragments of human SP-D having comparable immunological activities to native SP-D binds to gp120 and inhibit HIV-1 infectivity and replication in monocytic cells, Jurkat T cells and peripheral mononuclear blood cells inhibiting HIV-1 triggered cytokine storm. The immune surveillance role of SP-D in terms of its ability to recognise viruses and modulate unwanted inflammatory responses was considered broadly in relation to HIV-1 infection and SARS-Cov-2 diseases [21]. But there are no studies aimed at investigating the multiple HPV abnormalities at all in the lungs in the light of pulmonary pattern recognition receptors. As well as, nobody paid attention to the events predetermining carcinogenesis of the lungs in the case of HPV or HeLa cancer cells infectivities.
It was shown the result of inoculation of male mice with HeLa cells evidencing at least 10 times increase in the area of the testis in Figures 6 and 7 during a month. This might occur due to cell surface receptor transmembrane heparan sulphate proteoglycan syndecan-1, which is critically involved in the differentiation and propagation of various tumours [22]. The syndecan-1 protein is a receptor situated in the equatorial region of the sperm head that specifically binds to the papillomavirus coat protein L of the “late” expression and therefore has a poor influence on male fertility [23]. Possibly, this explains the active proliferation of HeLa cells and tumour overgrowths in the testis area. The oral vaccination with HPV16 E2 protein of infected male mice has a clearly detectable antiproliferative effect, causing a pronounced regression of testicular tumours in male mice.
The “early” expression HPV16 E2 protein has attracted the attention of other researchers [24]. By studying the structure and activity of the E2 protein, it was found that the E2 protein is a powerful repressor of the promoter from which the viral oncogenes HPV16 E6 and HPV16 E7 are transcribed. The E2 protein included two domains: a transactivation N-domain and a DNA-binding C domain. The N-terminal domain of the E2 protein is fused to a hinge, and the E8 gene product forms a complex called the E2C domain. The whole structure of E2C of joined E8^E2 proteins interacts with a common nuclear repressor complex in host cells. The dysregulation of the repressor complex causes different types of carcinogenesis [24].
During rapid infection of murine lungs, it might exist the receptor or complexes of proteins like well-known pattern recognition pathogen receptors on the surface area of alveolocytes. Toll-like receptors play an important role in the natural defences against virus infection. However, they may promote inflammation events related to cancer development. The interaction between TLR4/TLR9 gene polymorphisms and HPV16/18 infection influencing cervical lesion risk was shown in [25].
Different protease families are found on the plasma membrane in the secretory pathways of cells and widely expressed in the nasopharinx, respiratory tracts and so on, where they are involved in the tropism and pathogenesis of coronaviruses, influenza viruses and other respiratory viruses, hopefully as well as human papillomaviruses. Moreover, proteases play an important role in viral maturation by processing many polyproteins that are translated from the viral RNA [26], with the evidence obtained from various experiments supporting the notion that proteases can be a viable antiviral target for COVID-19. So proteases are promising drug targets for antiviral treatment (COVID-19 et cetera). Still, the drug development and therapeutics toward them could be a very complicated process taking into account the efficacy and toxicity of proteases modulating at the enzymatic, cellular, organ and as well as system levels [26]. The developing effective yet low-toxicity treatment and mild preventive therapies might be considered the use of L-amino acid oxidase or D-amino acid oxidase that have antibacterial, antiviral, anticancer and antiproliferative properties [27] and are showing highly immunogenic and oncolytic behaviour according to our data obtained with the treatment of murine lungs tumours with LAAO and DAAO. These results are worthy of attention and continuation of the investigation.
According to the above considered, the native loss of immune defence might be the reason for the missed immunity resulting to the rapid tumour formation at the next day of the placing of murine lungs in suspension of HeLa cells in our experiments.
5. Conclusion
Murine peripheral mononuclear blood cells, splenocytes and lungs were considered to be excellent experimental models to discover the immune response of cell immunity and the induction of immune T lymphocytes to different antigenic and therapeutic proteins. Furthermore, having adult healthy mice in the laboratory, one can appreciate the immune response of protein of interest during a week using the standard Elispot technique.
As it was found that murine lungs are very gentle of mice internal organs with a high inclination to tumour formation without strong defence by humeral or cells systems and are able very fast to form tumours overgrowths, murine lungs appeared to be the appropriate object for rapid screening of immune antigenic and oncolytic proteins, as well as substances with high oncogenic potentiality. Therefore, the whole procedure continued for 5–7 days in order to investigate the activity of proteins and therapeutics of interest.
| HPV type | Control | Experience | ||||
|---|---|---|---|---|---|---|
| IFN* | CD4* | CD8* | IFN* | CD4* | CD8* | |
| 16 | 128 | 108 | 72 | 1980, 1800, 1220 | 1120, 1182, 1230 | 1980, 2016, 1186 |
| 18 | 98 | 78 | 76 | 1240, 1450, 1560 | 1840, 1684, 1208 | 1360, 2000, 1820 |
| 31 | 240 | 132 | 208 | 1160, 1950, 1420 | 1760, 1620, 1800 | 1185, 1560, 1670 |
| 45 | 204 | 15 | 36 | 1680, 1720, 1850 | 1860, 1840, 1380 | 1960, 1820, 1920 |
Table A1.
The inductison of the synthesis of interferon (INF), CD4 and CD8 T lymphocytes in peripheral mononuclear blood cells from mice vaccinated with vaccines of antigenic HPV16, 18, 31 and 45 L1 by another antigenic protein of phylogenetically unrelated type of papillomavirus HPV6 L1.
Antibodies to INF, CD4 and CD8 T lymphocytes were used in analyses.
| Variant | γ-Interferon | CD4 | CD8 | Granzyme В | Perforin |
|---|---|---|---|---|---|
| Peripheral mononuclear blood cells (PMBC) | |||||
| Control | 5, 20, 17, 14 | 8, 9, 0 | 5, 4, 0 | 6, 7, 10, 7 | 0, 2, 5, 14 |
| +HeLa-E2 | 6, 10, 22, 3, 0, 51 | 7, 8, 4 | 12, 4, 14 | 8, 2, 8,17, 9, 5 | 41,6,4,7,5,8 |
| +HeLa+E2 | 420, 840, 584, 512, 576,460 | 615, 1232, 615 | 646, 1104 | 688, 924, 672, 840, 749, 720 | 760, 666, 1007, 552, 491, 354 |
| Splenocytes | |||||
| Control | 0, 6, 12, 0 | 1, 5 | 7, 0 | 6, 0, 0, 0 | 0,6,6, 0 |
| +HeLa-E2 | 9, 7, 0,14,43, 53 | 57, 25,16 | 9, 21, 7 | 14,44, 36, 28, 0,5 | 30, 15, 10 11, 14,11 |
| +HeLa+E2 | 448, 568, 664, 280, 680, 572 | 600,642, 595 | 511, 504, 336 | 570, 384, 540, 590, 564, 264 | 435, 485, 528, 618, 612, 552 |
Table A2.
Therapeutic action of HPV16 E2 on the content of γ-interferon, amount of CD4 and CD8 T lymphocytes, granzyme B and perforin in blood and spleen of mice, preliminary intramuscular injected with HeLa cells (amount of cells was presented in account per membrane nitrocellulose filter). Figures corresponded to individual mice (here and further 1 figure =1 mice).
| Variant | TCR | Granzyme B | Perforin | Granulysin |
|---|---|---|---|---|
| Peripheral mononuclear blood cells (PMBC) | ||||
| +HeLa -Е2 | 31, 20, 14, 27, 16, 23 | 33, 41, 15, 25,37, 39 | 44, 30, 9, 23, 25, 27 | 15, 19, 13, 20, 22, 25 |
| +HeLa +Е2 | 1224, 2512, 4688, 1892, 2800, 2312 | 1084, 1691, 4466, 4912, 5104, 6945 | 3532, 4280, 5120, 5216, 3248, 4096 | 5364, 4688, 4480, 4234, 5016, 4896 |
| Splenocytes | ||||
| +HeLa - E2 | 32, 40, 24, 10, 26, 9 | 40, 74, 59, 39, 45, 53 | 18, 15, 32, 20, 25, 26 | 18, 15, 13, 12, 19, 13 |
| +HeLa + E2 | 824, 1165, 864, 964, 896, 1176 | 1008, 1104, 634, 952, 1004, 1126 | 1480, 1304, 1392, 1384, 1064, 1392 | 1648, 1220, 992, 1360, 1088 |
Table A3.
The therapeutic action of HPV16 E2 on the content of T cell receptor (TCR), granzyme B, perforine and granulysine in peripheric mononuclear blood cells and splenocytes of mice, preliminary intramuscular injected with HeLa cells.
| Variant of IgG to: | Anti PD-1 IgG - E2 | Anti PD-L1 IgG - E2 | ||
|---|---|---|---|---|
| +HeLa | -HeLa | +HeLa | -HeLa | |
| The number of stained immunopositive colonies of Т lymphocytes (immune “spots”) | ||||
| TCR | 19, 20, 30, 40, 5 | 31 | 18, 27, 30, 38, 5 | 23 |
| INF | 11, 20, 25, 3, 2 | 29 | 23, 46, 35, 25, 17 | 25 |
| CD4 | 26, 16, 4, 20, 24 | 22 | 3, 16, 16, 12, 16 | 34 |
| CD8 | 21, 14, 7, 5, 15 | 15 | 55, 31, 29, 9, 19 | 20 |
| Anti D-1 IgG + HPV16 E2 | Аnti PD-L1 IgG + HPV16 E2 | |||
| TCR | 156, 174, 104, 105, 133 | 63 | 400, 210, 223, 304, 187 | 168 |
| INF | 185, 156, 147, 127, 63 | 31 | 169, 484, 195, 198, 238 | 260 |
| CD4 | 213, 228, 119, 106, 97 | 56 | 178, 145, 143, 274, 156 | 428 |
| CD8 | 169, 103, 170, 220, 127 | 96 | 210, 145, 195, 105,159 | 456 |
Table A4.
The content of colour immunopositive colonies of T lymphocytes isolated from mice lungs inoculated with the suspension of HeLa cells in the presence of antibodies to inhibitors of checkpoints PD-1 and PD-L1, activated by adding the effector HPV16 E2 from tomato fruit.
| IgG to: | +L-amino acid oxidase | Control* -LAAO | +D-amino acid oxidase | Control* -DAAO |
|---|---|---|---|---|
| INF | 84, 128, 96, 116, 88 | 10 | 168, 118, 108, 117, 140 | 37 |
| GrmB | 92, 128, 100, 138, 172 | 16 | 96, 108, 132, 128, 96 | 35 |
| Perf | 92, 112, 84, 92, 76 | 22 | 140, 125, 124, 92, 76 | 31 |
| Grnlys | 88, 104, 80, 104, 64 | 37 | 116, 292, 100, 104, 104 | 30 |
Table A5.
The activation of the synthesis of interferon (INF), granzyme B (Grm B), perforin (perf) and granulysin (Grnlys) in T lymphocytes isolated from mice periferic mononuclear blood cells PMBC) after the addition of L-amino acid oxidase (LAAO) or of D-amino acid oxidase (DAAO).
- Mice PMBC without any treatment used as control ones.
| IgG to: | + L-amino acid oxidase | Control* -LAAO | + D-amino acid oxidase | Control* -DAAO |
|---|---|---|---|---|
| INF | 163, 174, 224, 105, 165 | 14 | 201, 179, 85, 152, 268 | 42 |
| Grm B | 141, 243, 144, 144, 149 | 12 | 186, 190, 142, 155, 336 | 58 |
| Perf | 119, 132, 126, 126, 221 | 67 | 191, 164, 103, 120, 184 | 59 |
| Grnlys | 231, 134, 102, 102, 93 | 15 | 73, 160, 158, 176, 224 | 56 |
Table A6.
The activation of the synthesis of interferon (INF), granzyme B (Grm B), perforin (perf) and granulysin (Grnlys) in T lymphocytes (splenocytes) isolated from mice spleen after the addition of L-amino acid oxidase (LAAO) or of D-amino acid oxidase (DAAO).
- Isolated splenocytes from mice spleen without any treatment used as control ones.
| IgG to: | +L-amino acid oxidase | Control -LAAO | +D-amino acid oxidase | Control -DAAO |
|---|---|---|---|---|
| INF | 376, 504, 364, 372, 364 | 29 | 456, 146, 160, 380, 370 | 53 |
| GrmB | 392, 492, 444, 368, 268 | 37 | 160, 280, 380, 372, 332 | 58 |
| Perf | 670, 336, 268, 332, 332 | 47 | 288, 352, 560, 436, 516 | 61 |
| Grnlys | 392, 364, 336, 348, 348 | 38 | 216, 336, 445, 103, 236 | 45 |
Table A7.
The content of immunospots in T lymphocytes isolated from mice lungs inoculated with cancer HeLa after treating with L-amino acid oxidase (LAAO) or with D-amino acid oxidase (DAAO).
References
- 1.
Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007; 121 :1-14. DOI: 10.1111/j.1365-2567.2007.02587.x - 2.
Siegel RL, Hiller D, Wagle NS, Jemal A. Cancer statistics, 2023. CA: A Cancer Journal for Clinicians. 2023; 73 :17-48. DOI: 10.3322/caac.21763 - 3.
Poizot-Martin J, Henry M, Benhaim S, Obry-Roget V, Figarella D, Tamalet C. High level of HPV16 and 18 DNA level in anal swabs from male and female HIV-1 infected patients. Journal of Clinical Virology. 2009; 44 :314-317. DOI: 10.1016/jcv.2009.02.003 - 4.
Smirnov VS, Kudryavtseva TA. Treatment of human papillomaviruses infection in HIV-infected patients. Russian Journal of Infection and Immunity. 2021; 11 :79-84. DOI: 10.15789/2220-7619-TOH-1233 - 5.
Kumar BV, Connors T, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018; 48 :202-213. DOI: 10.1016/j.immuni.2018.01.007 - 6.
Strebel PM, Papania MJ, Gastanaduy PA, Goodson JL. Plotkin’s vaccines. Seventh ed. Amsterdam: Elsevier; 2018. pp. 579-618. e.21. DOI: 10.1016/B978-0-35761-6.00037-7 - 7.
Lythe G, Callard RE, Hoare RL, Molina-Paris C. How many TCR clonotypes does a body maintain? Journal of Theoretical Biology. 2016; 389 :214-224. DOI: 10.1016/j.jtbi.2015.10.016 - 8.
Tough DF, Sprent J. Lifespan of naive and memory T cells. Stem Cells. 1995; 13 :242-249. DOI: 10.1002/stem.5530130305 - 9.
Macallan DC, Borghans JAM, Asquith B. Human T cell memory: A dynamic view. Vaccine. 2017; 5 :1-12. DOI: 10.3390/vaccines5010005 - 10.
Garbuglia AR, Lapa D, Sias D, Capabiachi MR, Del Porto P. The use of both therapeutic and prophylactic vaccines in the therapy of papillomavirus disease. Frontiers in immunology. 2020; 1 . Article 18:1-14. DOI: 10.3389/fimmu.2020.00188 - 11.
Beltran ORC, Ledezma RR. MVA E2 therapeutic vaccine for marked reduction in likelyhood of recurrent respiratory papillomatosis. Head & Neck. 2019; 41 :657-665. DOI: 10.1002/hed.244777 - 12.
Salyaev R, Rekoslavskaya N, Stolbikov A. The new plant expression system for the creation of vaccines against papillomaviruses. Doklady. Biochemistry and Biophysics. 2019; 484 :52-54. DOI: 10.1134/S1607672919010150 - 13.
Salyaev R, Rekoslavskaya N, Stolbikov A. Synthesis of proteins encoded by the early genes E2, E6 and E7 of papillomavirus of type 16 in the plant expression system. Doklady. Biochemistry and Biophysics. 2018; 482 :271-274. DOI: 10.1134/S1607672918050113 - 14.
Salyaev R, Rekoslavskaya N. The use of the antigenic protein HPV6 L1 to induce the synthesis of interferon, CD4 and CD8 T lymphocytes, and granzyme B in blood and splenocytes of mice in order to develop a broad vaccine against dangerous papillomatoses. Doklady. Biochemistry and Biophysics. 2021; 500 :331-335. DOI: 10.1134/S160767292105015X - 15.
Salyaev R, Rekoslavskaya N, Stolbikov A. The antiproliferative effect of the “early” protein E2 of papillomavirus HPV16 on testis tumors of mice induced by the injection of HeLa cells. Doklady. Biochemistry and Biophysics. 2019; 488 :296-299. DOI: 10.1134/S16076729190028 - 16.
Salyaev R, Rekoslavskaya N, Stolbikov A. The induction of the synthesis of interferon, CD4 and CD8 T lymphocytes in blood and spleen of mice after oral vaccination with the “early” protein HPV16 E2. Doklady. Biochemistry and Biophysics. 2019; 488 :316-319. DOI: 10.1134/S1607672919050077 - 17.
Salyaev R, Rekoslavskaya N. The effect of “early” proteins E2, E6 and E7 of papillmavirus of high-risk cancerogenous type HPV16 on HeLa cells inducing tumour growth in mice lungs. Acta Biomedica Scientifica. 2022; 7 :260-276. DOI: 10.29413/ABS.2022-7.3.26 - 18.
Salyaev R, Rekoslavskaya N, Stolbikov A. New data on the influence of the HPV16 E2 early protein obtained in plant expression nanosystems on tumor tissues of female mice induced by injection of HeLa cancer cells in hip muscle. Nanotechnologies in Russia. 2020; 15 :516-512. DOI: 10.1134/S1995078020040138 - 19.
Schneider JL, Rowe JH, Garcia-de-Alba C, Kim CF, Sharpe AH. The aging lung: Physiology, disease and immunity. Cell. 2021; 184 :1920-2019. DOI: 10.1016/j.cell.2021.03.005 - 20.
Ji J, Sun L, Luo Z, Zhang Y, Xianzheng W, Liao Y, et al. Potential therapeutic application of pulmonary surfactant lipids in the host defence against respiratory viral infections. Frontiers in Immunology. 2021; 12 : Article 730022:1-8. DOI: 10.3389/fimmu.2021.730022 - 21.
Beirag N, Kumar C, Madan T, Shamji MH, Bulla R, Mitchell D, et al. Human surfactant protein D facilitates SARS COV-2 pseudotype binding and entry in DC-sign expressing cells and downregulates spike protein induced inflammation. Frontiers in Immunology. 2022; 488 :13. Article 960733:1-19. DOI: 10.3389/fimmu.2022.960733 - 22.
Szatmari T, Otvos R, Hjerpe A, Dobra K. Syndecan-1 in cancer: Implication of cell signalling, differentiation and prognostication. Disease Marker (Hindawi Publishing Corporation). 2015; 488 :1-13. Article ID 796052. DOI: 10.1155/2015/796052 - 23.
Foresta C, Patassini C, Bertoldo A, Menegazzo M, Francavilla F, Barzon L, et al. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS One. 2011; 6 :e15036. DOI: 10.1371/journal.pone. 0015036 - 24.
Dreer M, Fertey J, van de Poel S, Straub E, Madung J, Macek B, et al. Interaction of NCOR/SMRT repressor complexes with papillomavirus E8^E2C proteins inhibits viral replication. PLOS Pathogens. 2016; 12 :e1005556. DOI: 10.1371/journal.ppat.1005556 - 25.
Zhang C, Yang Z, Luo P, Ye M, Gong P, Gong Q, et al. Association of TLR4 and TLR9 gene polymorphism with the risk and progression of cervical lesions in HPV-infected women. Biomarkers in Medicine. 2023; 17 :133-142. DOI: 10.2217/bmm-2022-0702 Epub. 2023 Apr25 - 26.
Luan B, Huynh T, Cheng X, Wang H-R. Targeting proteases for treating COVID-19. Journal of Proteome Research. 2020; 19 :4316-4326. DOI: 10.1021/acs.jproteome.0c00430 - 27.
Lukasheva EV, Efremova AA, Treshalina EM, Arinbasarova AY, Medentzev AG, Berezov TT. L-amino acid oxidases: Properties and molecular mechanisms of action. Biochemistry (Moscow). Supplement Series B. Biomedical Chem. 2011; 5 :337-345. DOI: 10.1134/S199075081104007X