The targeted genes by the different channels for each kit used in the Mostaganem COVID-19 laboratory.
Abstract
Accurate and rapid diagnostic tests are critical for achieving control of COVID-19, a pandemic illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Diagnostic tests for COVID-19 fall into two main categories: molecular and antigen tests. The reverse transcription real-time-quantitative polymerase chain reaction (RT-qPCR) has become the gold standard for diagnosis of the COVID-19; however, this test has many limitations that include potential false-negative results and precarious availability of test materials. The isothermal PCR or Loop-Mediated Isothermal Amplification (LAMP) method has generated substantial interest as an alternative or complement to RT-qPCR, as some might be cheaper and easier to implement at the point of care. To our knowledge, it is the first Algerian study that wanted to compare two different molecular biology methods: RT-qPCR and LAMP for rapid diagnosis of COVID-19. In this review, we wanted to compare the CTs observed by RT-qPCR with those observed by LAMP in the same laboratory.
Keywords
- COVID-19
- SARS-Cov-2
- RT-qPCR
- LAMP
- threshold CT
1. Introduction
The large-scale transmission of viral diseases poses a serious threat to global public health. In recent decades, several viral epidemics have emerged with increasing frequency, including severe acute respiratory syndrome coronavirus (SARS-CoV) and, more recently, Middle East respiratory syndrome coronavirus (MERS-CoV). Recently, a novel coronavirus (SARS-CoV-2) emerged in Wuhan province in China. Therefore, a global pandemic affected the working and living conditions of billions of people worldwide in late December 2019, in Wuhan. It is known as COVID-19 (Coronavirus Disease 2019), and this novel coronavirus rapidly spreads through China and then many other countries globally. Consequently, the World Health Organization (WHO) has declared COVID-19 an international public health emergency and has given a very high-risk assessment on a global level.
The COVID-19 disease has clinical manifestations that highly vary from individual to individual, ranging from mild flu-like symptoms to sometimes life-threatening conditions. Hence, the accurate diagnosis of COVID-19 is challenging. Routine clinical diagnosis of this outbreak is confirmed by several laboratory detection methods, including nucleic acid amplification tests and serological techniques. However, in some patients, the transit of immunity is delayed due to individual risk factors such as older age and co-morbidities like diabetes, hypertension, cardiopathy, and obesity [1, 2].
The SARS-CoV-2 virus testing and corresponding human antibodies are essential for the COVID-19 diagnosis and treatment. It is important to underline that this screening was also considered a prerequisite for different economic and social activities such as international flights, offline work, access to shopping malls as well as sporting and social events. For this, an unimaginable number of rapid molecular tests for COVID-19 have been carried out in numerous medical institutions.
It is evident that the reverse transcription real-time-quantitative polymerase chain reaction (RT-qPCR) still remains a gold standard in the detection of the coronavirus, but an immediate need for alternative less expensive, more rapid tests is clear. As for COVID-19 testing, the majority of countries have performed many RT-qPCR tests per 1000 people by now. This puts an additional constraint on the country’s economy. RT-qPCR tests that constitute the predominant part of these numbers cost on average DA2000–5000 (£20–50) per test. Not to mention the costs of electricity, equipment maintenance, and salaries for staff. For this reason, some laboratories have considered adopting new techniques such as LAMP (Loop-Mediated Isothermal Amplification).
Precisely, several studies published in 2021 have suggested that RT-LAMP seems to be a diagnostic tool for COVID-19 as an alternative to RT-qPCR, especially in the acute symptomatic phase of COVID-19 [3, 4]. Then in 2022, a meta-analysis carried out on 19 studies showed that the LAMP method is faster and highly specific for the detection of SARS-CoV-2 compared to RT-qPCR [5].
In Algeria, the SARS-Cov-2 RT-qPCR test is available at the COVID-19 services of various hospitals, some university laboratories, and at all Algerian Pasteur Institutes. In addition, some Algerian COVID-19 services have adopted the LAMP technique, such as the COVID-19 Laboratory of the University of Mostaganem, Algeria. You should also know that the results of an RT-PCR or RT-LAMP test can be reported in viral load (CV) (x copies/ml) or in threshold cycle value (Ct) between 0 and 40. The values Ct are inversely correlated with CV and the correlation formula varies according to the diagnostic platform used. The aim of this study was first to compare the CTs found using the different RT-qPCR kits at the COVID-19 laboratory of the University of Mostaganem. Furthermore, we wanted to compare the CTs observed by RT-qPCR with those observed by LAMP in the same laboratory. To our knowledge, this is the first study that tries to compare these two important molecular techniques for the diagnosis of COVID-19 in Algeria.
2. Comparison test between RT-qPCR and LAMP in COVID-19 diagnosis
Coronavirus is a positive-sense single-stranded RNA virus. The viral envelope consists of a lipid bilayer where the membrane (M), envelope (E), and spike (S) structural proteins are anchored. A subset of coronaviruses (in particular, beta coronavirus) also have a shorter spike-like surface protein called hemagglutinin esterase (HE). Inside the envelope, there is the nucleocapsid, which is formed from multiple copies of the nucleocapsid (N) protein. This protein is bound to the single-stranded RNA genome. The lipid bilayer envelope, membrane proteins, and nucleocapsid protect the virus when it is outside the host cell [6, 7, 8, 9, 10, 11, 12, 13].
Some World Centers for Disease Control and Prevention rapidly employed molecular assays for the detection of COVID-19, mostly, employing the development of real-time polymerase chain reaction (RT-PCR) methods to diagnose COVID-19 [1]. Molecular tests for the SARS-Cov2 virus use improved quantitative RT-PCR methods characterized by rapid detection, high sensitivity, and specificity. These tests target various genetic combinations according to the already published protein and genomic structure of this virus. The genes mainly targeted are Open Reading Frame (ORF) gene, envelope (E) gene, nucleocapsid (N), and RNA-dependent RNA polymerase (RdRp) gene [14, 15, 16, 17, 18, 19]. However, other new PCR-based methods like the LAMP (Loop-Mediated Isothermal Amplification) method also show higher and improved specificity and test sensitivity.
2.1 The reverse transcription real-time PCR (RT-qPCR)
RT-qPCR is a technique that makes it possible to perform quantitative PCR from an RNA sample. RNA is first reverse transcribed using an enzyme called reverse transcriptase, which allows the synthesis of complementary DNA (cDNA). As in conventional PCR, the cycles of a conventional quantitative PCR reaction can take place in three steps: denaturation, hybridization, and elongation. However, in RT-qPCR, fluorescent labeling allows data collection as the PCR progresses, and it can be done in one or two steps. “One step” tests combine reverse transcription and PCR in a single tube and buffer. Regarding “two-step” RT-qPCR, reverse transcription and PCR are performed in separate tubes, with different optimized buffers.
To reduce the risk of false positives from the amplification of any contaminating genomic DNA, it is best to design primers for the qPCR step of RT-qPCR at a region that spans an exon-exon junction.
Ideally, all RT-qPCR experiments should include a negative reverse transcription control (−RT control) to test for contaminating DNA. Of course, like any PCR reaction, this control contains all of the reaction components except the reverse transcriptase. Therefore, if PCR amplification is observed, it is most likely derived from the contaminating DNA [20, 21].
2.2 The LAMP (loop-mediated isothermal amplification)
Loop-Mediated Isothermal Amplification (LAMP) is a novel isothermal nucleic acid amplification method. The classic PCR has been used for pathogen detection for more than 30 years. The process of this new technique uses high temperatures to denature the DNA, then the temperatures are cooled, and the primers bind to the DNA. Thus, to detect pathogens such as SARS-Cov-2, it is sufficient to program repeated cycles of high and low temperatures and primers for DNA amplification.
Unlike conventional PCR, LAMP uses four to six primers to recognize six distinct regions of DNA or RNA. LAMP primers cause displacement of the DNA strand and cause a loop to form at the end of the DNA strand. This structure allows the exponential accumulation of additional double-stranded DNA and is the basis for amplification [21].
Numerous studies have now shown the successful application of LAMP assays in various forms to detect coronavirus RNA in patient samples, demonstrating that 1–10 copies of viral RNA template per reaction were sufficient for successful detection, which were ~ 100-fold more sensitive than conventional RT-PCR methods [22, 23, 24, 25, 26, 27, 28]. The LAMP method does not contain a double-stranded DNA purification or denaturation step, which explains the speed of the analysis.
LAMP is currently one of the most widely used isothermal methods, and it has quickly taken its place in molecular biology laboratories, especially those that do not have PCR equipment. It is a simple and rapid DNA/RNA amplification method that takes place in a thermocycler or a water bath at 65°C. It was developed by
3. Comparison study between the kits used for the rapid diagnosis of COVID-19 in algeria
Importantly, different RT-qPCR protocols use different primer-probe sets targeting various segments of the SARS-CoV-2 genome. These protocols may not have the same analytical or clinical sensitivity and specificity, even when used for the same COVID-19 sample [30]. It should also be noted that different RT-qPCR kits designed for the detection of SARS-CoV-2 have different reagents. A recent study comparing the contents of four kits developed by different institutions (US CDC, China CDC, Germany CU, and the University of Hong Kong) showed that even when the tests are identical, their concentrations in the reaction mixture can be different [31, 32, 33]. Some researchers critically compared the analytical efficiencies and sensitivities of these four RT-qPCR assays, and each of them is likely to have different sensitivity/specificity, and possibly different accuracies. They concluded that all the primer-probe sets for these four assays can be used to detect SARSCoV-2 as long as the limitations of each assay are recognized (DANAFORM®). However, they noted that the different assays are capable to differentiate between true negatives and positives when a low load of SARS-CoV-2 is present in a given sample [34].
3.1 Study design
This is a retrospective study carried out at the level of the molecular biology laboratory of Mostaganem from January 01, 2021, to December 01, 2022.
Out of a total of 7021 samples received at our laboratory, only 6595 were retained. In our study, the samples were taken either in hospitals or in the universities of the province of Mostaganem. The exclusion criteria were noncompliant samples, incomplete information sheets, and inconclusive tests.
Viral RNA extraction was performed from the swab samples immediately in the COVID-19 laboratory of Mostaganem using the RNeasy Micro Kit (QIAGEN, GERMANY).
The samples were treated with nine different RT-qPCR kits: QuantiNova COVID-19-Imd, Da An Gene, Gene Proof, Sansure Biotech, Allplex, Cosaryon One Stepimd-, Thaiduong, and Snibe. The RT-qPCR was performed according to the protocols defined in respective technical sheets for each kit on two thermocyclers available in our laboratory (Bioer Line Gene Mini and QIAGEN Rotor-Gene Q).
In addition, the nasopharyngeal swab samples obtained from 33 COVID-19 patients were tested by RT-LAMP and that were already genotyped by RT-qPCR before. We started the study in January 2022 and unfortunately stopped when the reagents ran out.
Sensitivities and specificities of RT-LAMP compared with RT-qPCR were analyzed as a function of the CT values. The goal was to observe if there would be a noticeable difference between the results of the two methods.
For that, we used the “Smart Amp™ 2019 New Coronavirus Detection Reagent” co-developed by DNAFORM Corporation (Yokohama, Japan) and RIKEN (Yokohama, Japan) for LAMP. We also compared the RT-qPCR kits used for these 33 samples. These kits are QuantiNova COVID-19-Imd, Gene Proof, and Sansure Biotech. Afterward, we compared these three RT-qPCR kits with the LAMP Smart Amp kit.
3.1.1 QuantiNova kit (Germany)
The QuantiNova Multiplex RT-PCR Kit is intended for molecular biology applications. This product is not intended for the diagnosis, prevention, or treatment of a disease.
3.1.2 Sansure Biotech kit (China)
SARS-CoV-2 and Influenza A/B Virus Multiplex Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) (PCR-Fluorescence Probing). It is used for the detection of SARS-CoV-2 for two RdRp targets (IP2 and IP4).
3.1.3 Gene Proof SARS-CoV-2 PCR Kit (Czech)
The kit has been manufactured according to Directive 98/79/EC of the European Parliament as an in vitro diagnostic medical device and has been designed for professional use in clinical and specialized research laboratories. It detects the specific sequences of the virus genome (RdRp gene, E gene, and N gene) in a single reaction. The multiplex targeting mechanism ensures high sensitivity of SARS-CoV-2 detection.
3.1.4 Smart Amp SARS-CoV-2 Test (Russia)
The EMG Smart Amp SARS-Cov-2 RNA Detection kit is designed to detect the viral material presence in biological samples by implementing reverse transcription and real-time isothermal amplification reactions. It is characterized by a Fast Reaction Rate Typically 10 minutes–35 minutes, High Sensitivity From 100 copies, and High Specificity. It detects and identifies four target genes for SARS-CoV-2 using multiplex real-time PCR SARS-CoV-2 Assay.
3.2 RT-qPCR and RT-LAMP comparison
We have presented all the characteristics of the different kits used in the COVID-19 laboratory of the University of Mostaganem (Table 1).
| RT-PCR Kits | Reading channels | Time | CT values | |||
|---|---|---|---|---|---|---|
| FAM | ROX/TEXAS RED | CYS | JOE/VIC | |||
| RdRp | Nsp9 | / | IC | 123 min | 35 | |
| ORF | N | IC | / | 118 min | 40 | |
| N | / | / | IC | 76 min | 40 | |
| RdRp/E/N | / | / | IC | 120 min | 35 | |
| N | / | IC | ORF | 132 min | 40 | |
| N3 | N1 | IC | N2 | 149 min | 40 | |
| ORF | N | / | IC | 87 min | 40 | |
| / | / | IC | RdRp | 106 min | 35 | |
| RdRp | Nsp9 | / | IC | 120 min | 35 | |
| Smart cible | / | / | / | 45 min | 35 | |
Table 1.
FAM, ROX, CYS, JOE/VIC: reading qPCR channels. Open Reading Frame: ORF gene, Envelope: E gene, Nucleocapsid: N gene, the Non-structural protein 9: Nsp9 gene, and RNA-dependent RNA polymerase: RdRp gene. CI: Internal Control, min: minute, CT values: Cycle Threshold.
As shown in Table 1, all RT-qPCR kits are composed of probes that detect a maximum of two different genes of the Sars-Cov2 virus (detection channels: FAM, ROX/TEXAS RED, CYS, and/or JOE/VIC) except the Gene Proof kit which targets three genes with a single common probe (FAM). However, the Smart Amp Kit is an RT-LAMP assay that can perform both reverse transcriptase and isothermal DNA amplification reactions in a single reaction tube (Table 1). We have also mentioned the duration determined for each RT-qPCR or RT-LAMP kit.
Also, we have provided an evaluation of our RT-qPCR and RT-LAMP tests with their different indicators of positivity, i.e., cycle threshold values (Ct). Indeed, knowledge of diagnostic tests for COVID-19 highlights the need for local validation of positive–negative Ct cutoff values when establishing RT-qPCR assays for the detection of SARS-CoV-2.
The threshold cycle value (Ct) is the actual number of cycles required for the PCR to detect the virus. It gives an estimate of the likely amount of virus present in the original sample. If the virus was found in a small number of cycles (Ct value less than 35), it means that it was easy to find in the sample, and therefore the sample contained a large amount of it.
3.3 Statistical analysis
The Pearson’s Chi-square test (2x2 table) for data analysis was used for numerous parameters such as sensitivity, specificity, negative and positive tests, and CT values. A P value <0.05 was considered statistically significant.
Summary statistics in Tables 2 and 3 are presented as the mean and standard error of the mean. All statistical analyses were performed with SPSS (IBM.SPSS. v.22.). The graphical tables and histograms were designed by Microsoft Excel Software (for Windows.11).
| RT-qPCR (n = 33) | LAMP (n = 33) | |
|---|---|---|
| CT mean ± SE | 25.55 ± 7.14 | 23.04 ± 8.82 |
| P value | 0.23 | |
Table 2.
Comparison between all samples treated by the RT-qPCR kits with the LAMP kit.
N: number, CT: cycle threshold, SE: Standard Error. P-value <0.05 is statically significant.
| RT-PCR kits/Smart Amp kit | Mean CT | Standard Deviation | P value |
|---|---|---|---|
| 26.50 | 4.87 | 0.24 | |
| 18.86 | 9.52 | ||
| 37.01 | 3.95 | 0.26 | |
| 31.18 | 0.93 | ||
| 20.64 | 6.12 | 0.23 | |
| 27.22 | 4.39 |
Table 3.
Comparison between the 33 samples treated by the three RT-qPCR kits with the LAMP kit.
N: number of samples. CT: cycle threshold. SD: Standard Deviation. P value <0.05 is statically significant.
3.4 Ethic statement
The study was approved by the Institutional Review Board of the University of Mostaganem.
4. Results
RT-qPCR and RT-LAMP kits characteristics:
All the kits used in our laboratory are kept at −20 C ° except the Cosaryon One Step-IMD kit (kept at room temperature) and the Snibe kit (2 and 8°C) before its reconstruction.
Since the outbreak of the COVID-19 pandemic in 2020, we have observed that the Gene Proof kit has been used the most in our laboratory. But this is due only to the high availability of the latter.
As shown in Table 1, the Smart AMP kit is the only one that uses isothermal PCR, and suddenly it is the fastest test (45 minutes). In addition, concerning the RT-qPCR, the Thai Duong kit is the slowest test (149 minutes), while the Cosaryon test is the fastest (76 minutes).
Hoffmann’s meta-analysis also found a time variation ranging from 30 to 60 minutes, which in any case makes RT-LAMP an extremely fast method compared to RT-PCR, which requires about 120 to 160 min to complete [5].
The Ct value is influenced by several factors, including the PCR test kit used, the time of sample collection, the machine used for analysis, the technique used by the healthcare professional to obtain the sample, and the sample type (collection method). In our present work, CT threshold values were determined according to the validation methods of the COVID-19 laboratory in Mostaganem for each kit. Indeed, some RT-qPCR kits have a CT threshold = 35 and others = 40; however, the RT-LAMP (Smart Amp) kit has a CT threshold determined of 35 (Table 1).
Several studies have analyzed the effect of the CT threshold value on the diagnosis of COVID-19. Mainly, the study by Serrano-Cumplido et al. in 2021 [35] summarized the different possibilities of CT values obtained during an RT-qPCR reaction. The study was carried out on a sample of the Spanish population and concluded that CT values <30 determine an acute COVID-19 infection, CT values between 30 and 34 determine a moderate infection, and CT values between 34 and 37 are considered undetermined values. Finally, CT values >37 would rather indicate the absence of the SARS-Cov-2 virus. These threshold values can be slightly modified from one laboratory to another and according to the molecular kits used [35].
It should also be noted that all the kits used in this study target a maximum of two different genes (ORF gene, N gene, Nsp9 gene, and/or RdRp gene) except the Gene Proof kit target three different SARS-CoV-2 genes (RdRp gene, E gene, and N gene) at the same time with a common probe (FAM) (Table 1).
RT-qPCR and RT-LAMP kits comparison:
Otherwise, to achieve our goal of comparing the RT-qPCR and LAMP methods in the detection of the SARS-Cov-2 virus, we succeeded in comparing 33 samples determined by three RT-qPCR kits (QuantiNova, Sansure, and Gene Proof) with the Smart Amp kit of the LAMP method.
As shown in Table 2, we have observed that the mean of the CT thresholds obtained by the LAMP method of the 33 samples is lower than that obtained by the RT-qPCR. This difference was not statically significant (p > 0.05) (Table 2).
As shown in Table 3, we compared each sample determined separately by an RT-qPCR kit with the RT-LAMP (Smart Amp) kit. Again, we found no statistically significant difference between mean CT (p > 0.05).
Furthermore, we have noted that there is a strong difference in the average CTs between the kits. Indeed, the average CT between the two kits QuantiNova and Sansure is much higher than that obtained by LAMP (respectively 26.50 vs. 18.86 and 37.01 vs. 31.18). However, the average CT obtained by the Gene Proof kit is rather lower than that of Smart Amp (20.64 vs. 27.22, respectively).
The study by Inaba M et al. analyzed the sensitivity and specificity of the LAMP method compared to RT-qPCR. Inaba’s team showed that the sensitivity and specificity of RT-LAMP were 100%. This suggests that the LAMP method has the same reliability as RT-qPCR [3].
On the other hand, the following figures demonstrate and confirm these observations regarding the use of the two COVID-19 detection techniques in Algeria (Figures 1–3).
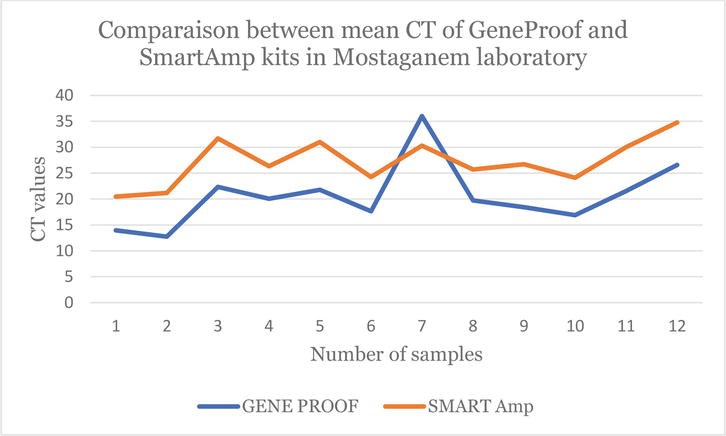
Figure 1.
Line chart which shows the difference between the CT values obtained by the
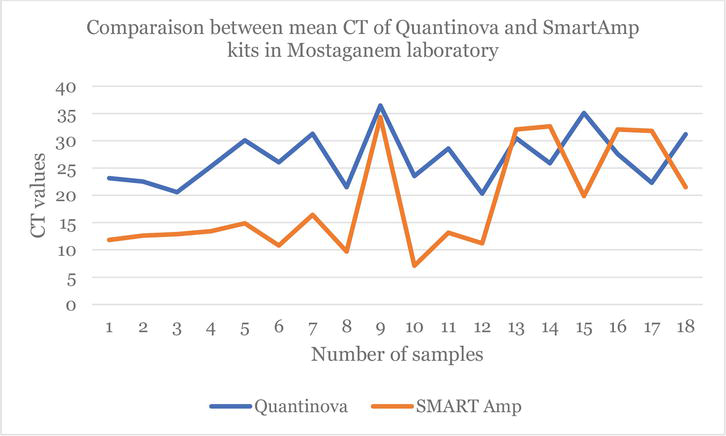
Figure 2.
Line chart which shows the difference between the CT values obtained by the
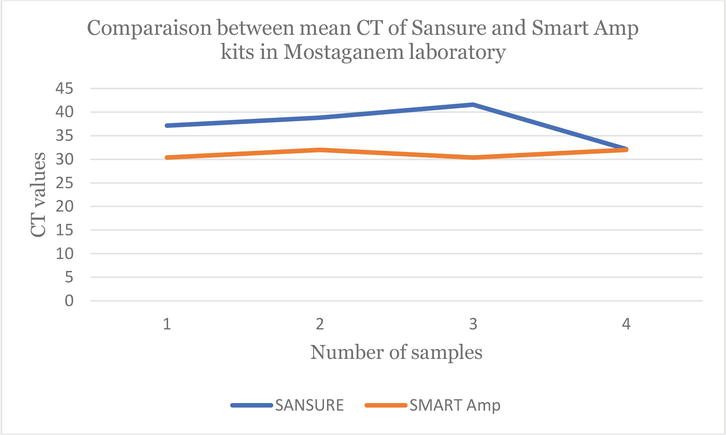
Figure 3.
Line chart which shows the difference between the CT values obtained by the
According to Figure 1, we can confirm that the CT values obtained by the Gene Proof kit (Blue line) are better than the CT values obtained by the Smart Amp kit (Orange line). Also, only one sample found to be COVID-19 negative (CT = 36.02) by RT-qPCR was found COVID-19 positive (CT = 30.32) by the LAMP.
Figures 2 and 3 show the difference between the CT values obtained by the two RT-qPCR kits and those obtained by isothermal PCR. It is clear that the orange lines are lower than the blue lines (Figures 2 and 3). This once again confirms the effectiveness of the LAMP method in the detection of the SARS-Cov-2 virus. Moreover, several negative COVID-19 cases by the RT-qPCR kits (QuantiNova and Sansure) were found to be rather COVID-19 positive by the LAMP Smart Amp kit. This suggests that the LAMP method can identify individuals determined to be “COVID-19 negative” by RT-qPCR as “false negatives”.
Although the Ct value does not indicate the severity of the disease, it could play an important role in clinical and public health decision-making if carefully analyzed along with other factors such as the type of test used, the history of exposure to COVID-19, symptoms, and individual patient characteristics. This analysis is done by health professionals and laboratories, who fully understand all the factors under study [35].
The efficiency of the LAMP is logical given the detection principle developed for this method using a set of six primers that target eight regions of SARS-Cov-2. In addition, the Gene Proof kit also has the ability to target three SARS-Cov-2 genes using a common probe, and this explains the results obtained by comparing it with the LAMP Smart Amp kit. We concluded that the LAMP with its many advantages (speed, simplicity, etc.) can replace RT-qPCR in the search for SARS-Cov-2, especially when the kits on the market are not adapted to a given ethnic population.
5. Conclusion
It cannot be denied that the RT-qPCR diagnostic assays used have high sensitivity and specificity, making them the gold standard for COVID-19 diagnosis. In addition, it must be emphasized that the different kits developed for the RT-qPCR method to detect SARS-Cov-2 use different sets of primer probes targeting various segments of the SARS-CoV-2 genome.
These kits/tests have different sensitivity/specificity and possibly different accuracies. However, it is a lengthy method and requires complex equipment and a highly skilled operator. In order to safely return to normal life, a test needs to be rapid, portable, and have enough analytical sensitivity to maintain a 90% or higher true positive rate. Loop-Mediated Isothermal Amplification (LAMP) is a method of nucleic acid amplification that exhibits increased sensitivity and specificity that are significantly rapid and do not require expensive reagents or instruments, which aids in cost reduction for coronavirus detection.
Since the start of the pandemic, the real-time RT-qPCR test has been the gold standard for diagnosing SARS-CoV-2. Unfortunately, cases of false negatives have also been reported due to different issues: sample collection, sample transport, and viral RNA extraction. Indeed, RT-qPCR assays have many limitations such as a high workload that requires skilled operators for testing and sample collection as well as expensive instruments and well-equipped laboratories.
In this study, we wanted to compare these two molecular biology methods capable of detecting SARS-Cov-2 in Algeria. Indeed, we amplified the same sample taken at random from the COVID-19 laboratory in Mostaganem (already determined by RT-qPCR) by isothermal PCR (LAMP) using the Smart Amp kit. Even though our sample size is not large, LAMP is an ultrasensitive nucleic acid amplification method that can detect minute quantities of DNA or RNA templates within roughly an hour, far outstripping normally utilized RT-PCR methods, particularly with the current demands for rapid and sensitive testing.
This original study is only an initial part of a long work that remains to be done by our team at the University of Mostaganem. It would be interesting to increase the size of the sample and that other laboratories also get involved.
Acknowledgments
We would like to thank
References
- 1.
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. European Communicable Disease Bulletin. 2020; 25 :30 Epub 2020/01/30 - 2.
Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. Journal of Virology. 2020; 94 :7 Epub 2020/01/31 - 3.
Inaba M, Higashimoto Y, Toyama Y, Horiguchi T, Hibino M, Iwata M, et al. Diagnostic accuracy of LAMP versus PCR over the course of SARS-CoV-2 infection. International Journal of Infectious Diseases: IJID: Official publication of the International Society for Infectious Diseases. 2021; 107 :195-200 Epub 2021/04/17 - 4.
Baba MM, Bitew M, Fokam J, Lelo EA, Ahidjo A, Asmamaw K, et al. Diagnostic performance of a colorimetric RT-LAMP for the identification of SARS-CoV-2: A multicenter prospective clinical evaluation in sub-Saharan Africa. EClinicalMedicine. 2021; 40 :101101 Epub 2021/09/04 - 5.
da Rosa E, Hoffmann TMD, Ghiotto MA, Gaboardi G, Cantarelli VV. Performance of loop-mediated isothermal amplification (LAMP) targeting the Nucleocapsid (N) gene of SARS-CoV-2 for rapid diagnosis of COVID-19. Systematic Review and Meta-Analysis. COVID. 2022; 2 :759-766 - 6.
Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proceedings of the National Academy of Sciences of the United States of America. 2009; 106 (14):5871-5876 Epub 2009/03/27 - 7.
Bosch BJ, van der Zee R, de Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein: Structural and functional characterization of the fusion core complex. Journal of Virology. 2003; 77 (16):8801-8811 Epub 2003/07/30 - 8.
Alic S, Dermastia M, Burger J, Dickinson M, Pietersen G, Dreo T. Genome-informed design of a LAMP assay for the specific detection of the strain of 'Candidatus Phytoplasma asteris' Phytoplasma occurring in grapevines in South Africa. Plant Disease. 2022; 106 (11):2927-2939 Epub 2022/04/06 - 9.
Kirchdoerfer RN, Cottrell CA, Wang N, Pallesen J, Yassine HM, Turner HL, et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016; 531 (7592):118-121 Epub 2016/03/05 - 10.
Millet JK, Whittaker GR. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Research. 2015; 202 :120-134 Epub 2014/12/03 - 11.
Tortorici MA, Veesler D. Structural insights into coronavirus entry. Advances in Virus Research. 2019; 105 :93-116 Epub 2019/09/17 - 12.
de Groot RJ. Structure, function and evolution of the hemagglutinin-esterase proteins of corona- and toroviruses. Glycoconjugate Journal. 2006; 23 (1-2):59-72 Epub 2006/04/01 - 13.
Neuman BW, Kiss G, Kunding AH, Bhella D, Baksh MF, Connelly S, et al. A structural analysis of M protein in coronavirus assembly and morphology. Journal of Structural Biology. 2011; 174 (1):11-22 Epub 2010/12/07 - 14.
Binnicker MJ. Emergence of a novel coronavirus disease (COVID-19) and the importance of diagnostic testing: Why partnership between clinical laboratories, public health agencies, and industry is essential to control the outbreak. Clinical chemistry. 2020; 66 (5):664-666 Epub 2020/02/23 - 15.
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine. 2020; 382 (8):727-733 Epub 2020/01/25 - 16.
Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. The New England Journal of Medicine. 2020; 382 (10):929-936 Epub 2020/02/01 - 17.
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579 (7798):270-273 Epub 2020/02/06 - 18.
Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clinical Chemistry. 2020; 66 (4):549-555 Epub 2020/02/08 - 19.
Shen M, Zhou Y, Ye J, Abdullah Al-Maskri AA, Kang Y, Zeng S, et al. Recent advances and perspectives of nucleic acid detection for coronavirus. Journal of Pharmaceutical Analysis. 2020; 10 (2):97-101 Epub 2020/04/16 - 20.
Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. Journal of Biomolecular Techniques: JBT. 2004; 15 (3):155-166 Epub 2004/08/28 - 21.
A. M. LAMP-Based Testing for COVID-19. Technology/Networks Industry Insight; 2020 - 22.
Poon LL, Leung CS, Tashiro M, Chan KH, Wong BW, Yuen KY, et al. Rapid detection of the severe acute respiratory syndrome (SARS) coronavirus by a loop-mediated isothermal amplification assay. Clinical Chemistry. 2004; 50 (6):1050-1052 Epub 2004/04/01 - 23.
Pyrc K, Milewska A, Potempa J. Development of loop-mediated isothermal amplification assay for detection of human coronavirus-NL63. Journal of Virological Methods. 2011; 175 (1):133-136 Epub 2011/05/07 - 24.
Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochemical and Biophysical Research Communications. 2001; 289 (1):150-154 Epub 2001/11/16 - 25.
Shirato K, Yano T, Senba S, Akachi S, Kobayashi T, Nishinaka T, et al. Detection of Middle East respiratory syndrome coronavirus using reverse transcription loop-mediated isothermal amplification (RT-LAMP). Virology Journal. 2014; 11 :139 Epub 2014/08/12 - 26.
Hong TC, Mai QL, Cuong DV, Parida M, Minekawa H, Notomi T, et al. Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. Journal of Clinical Microbiology. 2004; 42 (5):1956-1961 Epub 2004/05/08 - 27.
Njiru ZK. Loop-mediated isothermal amplification technology: Towards point of care diagnostics. PLoS Neglected Tropical Diseases. 2012; 6 (6):e1572 Epub 2012/06/30 - 28.
Shirato K, Semba S, El-Kafrawy SA, Hassan AM, Tolah AM, Takayama I, et al. Development of fluorescent reverse transcription loop-mediated isothermal amplification (RT-LAMP) using quenching probes for the detection of the Middle East respiratory syndrome coronavirus. Journal of Virological Methods. 2018; 258 :41-48 Epub 2018/05/16 - 29.
Craw P, Balachandran W. Isothermal nucleic acid amplification technologies for point-of-care diagnostics: A critical review. Lab on a Chip. 2012; 12 (14):2469-2486 Epub 2012/05/18 - 30.
Kawai Y, Kimura Y, Lezhava A, Kanamori H, Usui K, Hanami T, et al. One-step detection of the 2009 pandemic influenza A(H1N1) virus by the RT-SmartAmp assay and its clinical validation. PLoS One. 2012; 7 (1):e30236 Epub 2012/02/02 - 31.
Jeong YJ, Park K, Kim DE. Isothermal DNA amplification in vitro: The helicase-dependent amplification system. Cellular and Molecular Life Sciences: CMLS. 2009; 66 (20):3325-3336 Epub 2009/07/25 - 32.
Asai N, Nakamura A, Sakanashi D, Koita I, Ohashi W, Kawamoto Y, et al. Comparative study of SmartAmp assay and reverse transcription-polymerase chain reaction by saliva specimen for the diagnosing COVID-19. Journal of Infection and Chemotherapy: Official Journal of the Japan Society of Chemotherapy. 2022; 28 (1):120-123 Epub 2021/09/29 - 33.
Yamamoto K, Ohmagari N. Microbiological testing for coronavirus disease 2019. JMA Journal. 2021; 4 (2):67-75 Epub 2021/05/18 - 34.
Nagasawa S, Mori A, Hirata Y, Motomura A, Ishii N, Okaba K, et al. SmartAmp method can rapidly detect SARS-CoV-2 in dead bodies. Forensic Science International. 2022; 331 :111168 Epub 2022/01/10 - 35.
Serrano-Cumplido A, Ruiz Garcia A, Segura-Fragoso A, Olmo-Quintana V, Mico Perez RM, Barquilla-Garcia A, et al. Application of the PCR number of cycle threshold value (Ct) in COVID-19. SEMERGEN. 2021; 47 (5):337-341 Epub 2021/06/23. Aplicacion del valor umbral del numero de ciclos (Ct) de PCR en la COVID-19