Abstract
Diabetes (DM) is a significant risk factor for the onset and development of late diabetic complications at any age. In the elderly, DM often occurs as part of multimorbidity and can contribute to the onset and development of disability. The treatment of DM in old age is based on the same principles as for younger individuals. When choosing therapy for DM, the following should be taken into account: age, life expectancy, the presence of complications, self-sufficiency, economic conditions, eating habits and other handicaps. The authors report their own experience from the outpatient practice of DM type 2 treatment. The authors discuss the growth of the elderly population in relation to organ changes with the ageing process, as well as issues of multimorbidity, the specifics of the clinical picture of diseases in old age and the problem of polypharmacy both from the perspective of ageing and old age and the relationship to diabetes as a comorbidity.
Keywords
- aging
- diabetes in old age
- geriatrization of medicine
- multimorbidity
- organ specifics and diseases particularities
- polypharmacy
1. Introduction
Currently, apart from aging of the population, we are witnessing pandemics of type 2 diabetes (T2DM) and obesity, not only in industrialized countries but literally worldwide [1]. The prevalence of diabetes has doubled since 1980, and similarly, obesity has almost tripled between 1975 and 2020 [2].
Diabetes (DM) represents a significant risk factor for the emergence and development of late diabetic complications, both micro- and macroangiopathic [3]. In the elderly, DM often occurs as part of multimorbidity and can contribute to the onset and development of disability [4]. This can then substantially influence all the other diagnostic and treatment procedures. The treatment of DM in old age is based on the same principles as in younger individuals: diet, physical activity and possible medicines. For these reasons, I will not discuss DM therapy in detail and will leave this to other co-authors. On the contrary, I will focus on some specifics for DM only in old age.
Civilization development increases the hope of reaching old age, and the average life expectancy grows. Until the 1950s, people died prematurely. Over the past 100 years, life expectancy has almost doubled, which is one of the greatest achievements of humanity and science ever. The length of human life is now beginning to approach its biological limit. The probability of living in old age ceases to be an exceptional phenomenon but becomes a normal and mass matter.
The number of old, very old and long-lived people is increasing both absolutely and relatively [5, 6]. This trend will continue and become significantly stronger with aging of the baby boomers (people born after World War II) [6, 7, 8]. A basic overview and knowledge of the issues of geriatric medicine will become a necessity. Contemporary geriatric medicine strives to maintain adequate physical and mental activity for as long as possible, to prevent the loss of self-sufficiency and to improve the prognosis of the elderly in case of illness. It, therefore, has an interventional preventive character and supports successful healthy aging [9]. In the chapter, on the contrary, I will try to point out the specifics of organ changes and diseases in old age, which are fully applicable to older diabetics as well. For these reasons, in the text of the chapter, I focus on the issue of the peculiarities of geriatric morbidity and pharmacotherapy in old age (with emphasis on DM).
Demographic forecasts, not only in industrially advanced countries but also in third-world countries, point to a clear trend of global population aging. Understandably, this fact brings a number of new, not yet common and fully identified problems, both in the field of health and social care [10]. The length of life itself ceases to be the main parameter, and the quality of life of an older individual comes to the fore [11].
The senior population as a whole is an extremely heterogeneous group where there are both sick and healthy people [12, 13]. The expected volume of costs in the coming decades is estimated to reach almost twice the current level when it exceeds 40%.
The determination of age bands for old age is a convention and a social construction resulting from the administrative needs of the welfare state. Today, the age of 65 is generally considered to be the beginning of old age, and old age proper (in the narrower sense of the word) is spoken of from 75. At the same time, the most used new age classification of seniors is also derived from this concept:
65–74 years: “young old seniors”, issues of retirement, free time, activities, self-realization dominate.
75–84 years: “old seniors”, issues of adaptation, load tolerance, specific moaning, loneliness. Age over 75 years – more significant changes associated with physiological aging develop.
85 years and older: “oldest old seniors”, usually set aside as a separate category due to the high number of frail seniors and the high risk of the possibility of sudden addiction.
Demographic prognosis clearly indicates the rise of numbers of seniors with limited self-sufficiency or even “fragile” (frailty syndrome) [14, 15, 16, 17], who require a specific approach both in the health field (including pharmacotherapy) and in the social sphere [18, 19]. Life expectancy in the middle of the twenty-first century will reach 85–90 years. Seniors at this age will have depleted physiological reserve mechanisms (to a certain extent) such as immunity, adaptability and functional fitness [19].
Similarly, as society is aging, medical science is currently witnessing a phenomenon – the “geriatrization of medicine”. This fact is already significantly reflected in all branches of medicine today – starting with the first line (general practitioners) and ending with almost all specialists. The trend of population aging must be understood as a positive phenomenon of the twenty-first century, as it is a consequence of longer life and better health [1]. Future seniors will very soon also have other demands and requirements for health, social and other community services, which will have to adapt more and more to their needs [20].
The current and future challenges of medicine in the care of the elderly will mainly be:
Atomization of medicine
Absence of a holistic approach
Multimorbidity of the elderly, when they seemingly do not belong anywhere or in many different places
Excessive socialization of health problems
2. Specificity of diabetes in old age
Diabetes mellitus (DM) affects up to 20% of people in the 7th decade of age, and another 20% have impaired glucose tolerance. In the elderly it is mostly DM type 2 (over 70 years up to 95%). Less frequently, in old age, we can encounter DM type 1, which people bring with them from earlier years. DM1 can, however, also occur in old age, and not only the Latent Autoimmune Diabetes of Adults (LADA) type, where it is an autoimmune insulitis that has been going on for years. Diabetics over 65 years old with DM duration of more than 10 years have more comorbidities and late complications of DM. This fact only proves the huge heterogeneity of the population of older diabetics, where, on the one hand, there will be people with type 1 diabetes with DM duration of several decades and advanced organ complications; on the other hand, a large group of people where T2DM is usually diagnosed in the 7th or 8th decade without corresponding organ complications. Some of them will be fully able to manage their disease, while others will not due to cognitive dysfunction visual or other handicaps. The management of DM in old age must fully respect phenomena such as the duration of diabetes, complications and comorbidities, the chance of survival, the patient’s preferences and the functional capacity of a particular senior.
Significant differences in the characteristics of diabetes in the middle and younger ages as compared to the elderly mainly are:
Worse social-economic situation
Greater social isolation and loneliness
Greater need for help and dependence on family, caregivers, etc.
Polypharmacy
Frequent occurrence of senile frailty syndrome and limited life expectancy
Significant comorbidities that limit the senior’s independence
Lower ability to recognize hypoglycaemia and perform self-monitoring
Correct screening is the key to avoiding diabetic complications, morbidity and mortality.
Early diagnosis of DM of seniors in institutional care (nursing homes, etc.) can be of fundamental importance for their care [21].
Improvement of metabolic control can improve cognitive functions and reduce the risk of hypoglycaemia on the one hand and hyperosmolar coma on the other.
Early treatment can slow down the development of vascular complications and disability.
Properly managed dietary and regimen measures can also delay the need for medical treatment.
Treatment can relieve symptoms of DM and can improve quality of life.
3. Targets of DM treatment in old age
The objectives of DM compensation will be fundamentally different for the elderly without other serious handicaps and for the frail ones [22]. Achieving euglycaemia in elderly diabetics can often be unrealistic and sometimes even dangerous.
Pharmacologic treatment of DM and effective glycaemic control using appropriate and effective procedures can greatly alter the impact of the disease on comorbidities associated with micro- and macrovascular complications of the disease [23]. Figure 1 depicts the updated approach to DM2T therapy by the Professional Practice Committee (from European Association for the Study of Diabetes (EASD) and American Diabetes Association (ADA) recommendations) in relation to the degree of motivation, cooperation and self-sufficiency. It lists individual groups of antidiabetic drugs in relation to atherosclerosis-related cardiovascular (CVD) diseases (ASCVD); chronic kidney diseases (CKD) and heart failure (HF); further hypoglycaemia; possible weight gain or loss, and from a price point of view.
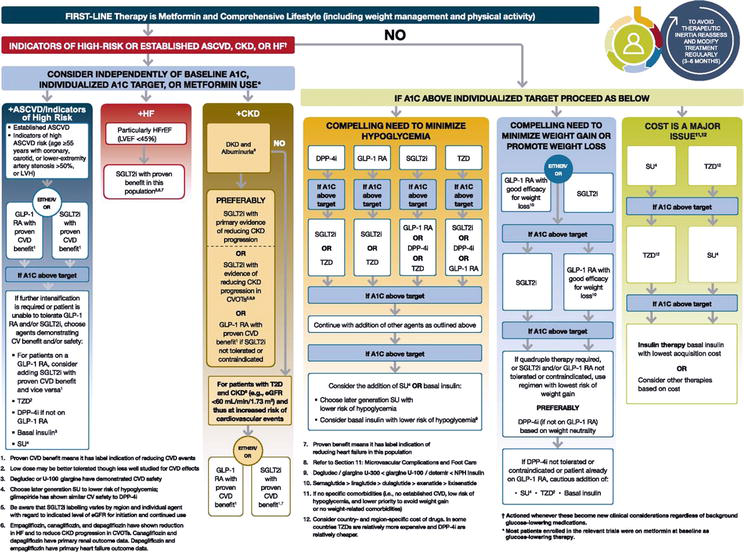
Figure 1.
Updated approach to DM2T therapy by the “professional practice committee (PPC)” of the American Diabetes Association -ADA considering complications, risk of hypoglycaemia, weight changes and cost.
In 2021, in our diabetological outpatient clinic Ltd.- outpatient departement (DIASTOP) of people with DM2T (2500 persons) we used: diabetic diet only 12%; oral antidiabetics (OAD) 53%; monotherapy with insulin or insulin analogues 17% and a combination of insulin and OAD (18%) – Figure 2 [24].
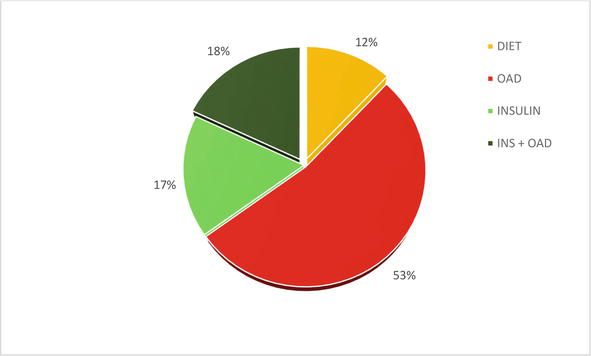
Figure 2.
Basic overview of DM2T treatment used – in 2021: Only diabetic diet; OAD = oral antidiabetic drug; INS insulin (CIT = classic insulin therapy and IIT = intensified insulin therapy); insulin + OAD.
Figure 3 shows changes in the use of the diabetic diet itself, sulfonylureas – SU (decrease in both) and metformin (MET) in 2008–2019 [25].
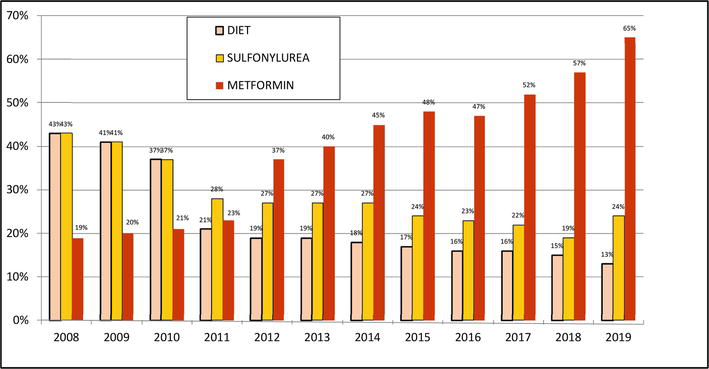
Figure 3.
Changes in the use of diabetic diet alone, sulfonylurea (SU) and metformin (MET) in 2008–2019.
New Antidiabetic Drugs (NAD) (gliptins, gliflozins and incretin mimetics) were used either within monotherapy or in combination in persons with DM2T in 2021 in a total of 35%. Our retrospective data analysis showed a steady trend in increasing NAD (most gliptins) use – shown in Figures 4–6 [24].
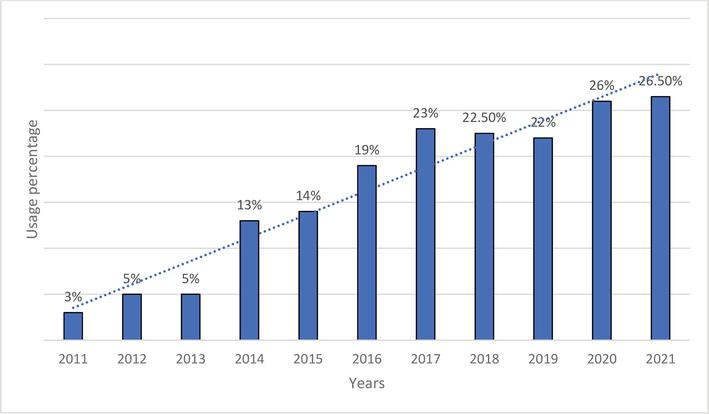
Figure 4.
Changes in the use of DPP4-inhibitors in 2011–2021.
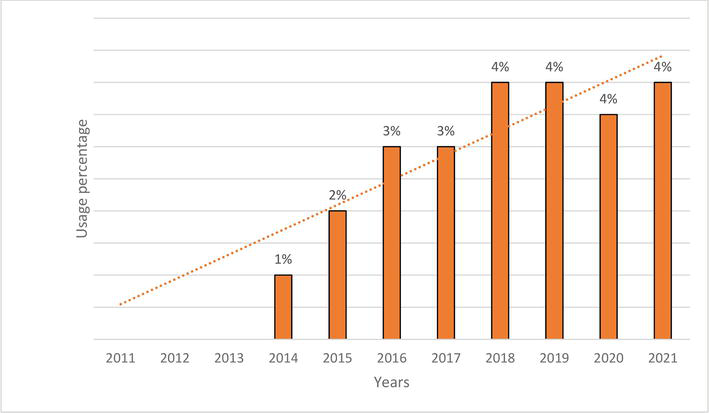
Figure 5.
Changes in the use of SGLT2-inhibitors in 2011–2021.
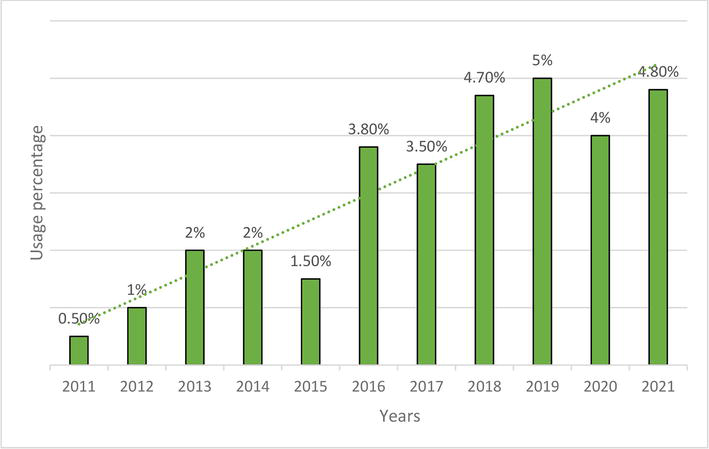
Figure 6.
Changes in the use of GLP1-agonists in 2011–2021.
It is not well known whether effective management of DM can improve the diverse impairments, i.e., functional, physical, cognitive, nutritional status and social exclusion, that are often associated with the presence of DM in old age. Therapeutic goals of the treatment of elderly people with DM have been summarized in the guidelines of the European Union Geriatric Medicine Society and the International Diabetes Foundation and must be consistently applied individually based on the functional status and the presence of comorbidities in elderly persons [26]. It is generally accepted that:
elderly persons without physical or cognitive disabilities should be treated in a similar way as the adult population.
glycaemic control in elderly persons with physical or cognitive disabilities or with a low life expectancy should be aimed at preventing symptomatic hyperglycaemia and the risk of hypoglycaemia [27]. Considerations when initiating DM therapy must include consideration of hypoglycaemia risk, comorbidities, current drug therapy, body mass and DM compensation options [28, 29, 30, 31]. Therapeutic options for T2DM in the elderly are diet, physical activity, various antidiabetic drugs, insulin and education [32, 33, 34]. When choosing an appropriate treatment, it is always necessary to take into account the age of the diabetic (including the chance of survival), macro and microangiopathic complications, the level of self-sufficiency, a family environment and economic situation, catering habits (including the state of the teeth) and other handicaps such as disabilities – mental, motor, sight and hearing deficits.
The European Diabetes Working Party for Older, in the published guidelines for the treatment of people with diabetes aged 70+ [26], recommends modifying the HbA1c target values with regard to age and comorbidities. The range HbA1c of 54–59 mmol/mol IFCC (7–7.5% DCCT) is recommended for elderly people with type 2 diabetes without major comorbidities and 60–70 mmol/mol IFCC (7.6–8.5% DCCT) for frail seniors = frail (not self-sufficient, multimorbid, with dementia), where the risk of developing hypoglycaemia is high and the benefit of medical intervention is relatively low. One of the main principles is to reduce the risk of hypoglycaemia, no diabetic should have fasting blood glucose <6.0 mmol/l during treatment. It should never be started if fasting blood glucose is consistently around 7.0 mmol/l. Low glycaemia (glucose levels <5.0 mmol/l) should never be treated with medication, and similarly, random blood glucose levels >11.0 mmol/l as well.
4. Geriatric syndromes: Coexistence with diabetes
Apart from typical acute and late specific and non-specific complications, which I will not discuss here, the emergence and development of typical geriatric syndromes come to the fore in the senior population [3, 4].
DM increases the risk of developing geriatric syndromes – such as dementia, depression, urinary incontinence, falls and a general decline in functional capacity [3, 6, 9].
4.1 Cognitive disorders
DM increases the risk of Alzheimer’s dementia by 50 to 100% and vascular dementia by 100 to 200%. Although, on the one hand, some works point to the fact that hyperglycaemia and poor DM control increase the risk of dementia, there are also studies showing that hypoglycaemia as a consequence of tight DM compensation similarly increases the risk of developing mental deterioration. Taking into account that the focus of effective DM treatment is self-monitoring, this will be a limiting factor even in persons with mild cognitive impairment, who will have a problem with adherence to regimen measures, diets and medication and will be unable to recognize potential hypoglycaemia.
4.2 Depression
Depression, as such, itself is associated with adverse side effects, including poorer quality of life, a decline of functional capacity and increased risk of death. Diabetes and depression coexist in 30% of individuals with DM. In 5–10% of diabetics, it can be a severe form of depression. Depression will worsen compliance and adherence to treatment, possibly even self-monitoring as well.
4.3 Urinary incontinence
MI is a problem for 50% of older women with DM. Urgent incontinence is three times higher, and stress incontinence is twice as high. Similar data are not available for men. A higher degree of obesity is also a risk factor for MI; conversely, reducing excess weight reduces the incidence of MI.
4.4 Falls and fractures
In general, individuals with a higher weight have DM more frequently but also a higher bone density, which is associated with a lower risk of fractures. Yu Yang [35] describes a greater risk of falls in older diabetics, and this association is more pronounced in insulin-treated diabetics compared to non-diabetics. The use of insulin, possibly hypoglycaemic insulin secretagogues, poorer eyesight and peripheral neuropathy, belong to the causes of a higher prevalence of falls in older people with DM [36].
5. Changes of organ systems in the elderly
The following paragraphs summarize examples of more serious physiological changes observed in older age people according to individual organ systems [37, 38, 39]. These will significantly influence morbidity in old age – including diabetes [8].
Loss of maximum breathing capacity decreases by about 40%. At the level of the alveoli, the exchange of oxygen and carbon dioxide decreases by about 50% between the ages of 30 and 65. Although these changes are not observable at rest, older individuals experience fatigue or shortness of breath during increased exertion (exercises or serious illness). Pulmonary reflexes such as cough (ciliary function) decrease with age and thus predispose older people to an accumulation of secretions and development of aspiration pneumonia.
Arterial pressure of oxygen (PaO2) decreases with age over a fairly wide range as a result of ventilation and perfusion imbalance. The reduced ventilatory response to hypoxia and hypercapnia is demonstrable in the elderly and is thought to be a decline in chemoreceptor function. All these changes do not lead to fundamental changes in resting oxygen saturation, but there is a decrease in arterial PaO2. The arterial PaO2 of many people over 80 is around 70-75 mmHg. These changes in blood gases are of minimal importance under resting conditions but dramatically affect survival during severe respiratory disease.
The age will limit the ability to respond correctly to the load with the compensatory mechanisms of the lungs and kidneys. For example, the ability of the lungs to respond by hyperventilating to acute metabolic acidosis is weakened and only leads to a further drop in pH. Aging kidneys also respond more slowly to acid load, and blood pH recovers more slowly. Many diseases that are frequent in older age (such as heart failure, anaemia, sepsis, diabetes mellitus, kidney and lung diseases) can overload regulatory systems and contribute to the development of acid-base disorders. Similarly, numerous drugs often used in senium (nonsteroidal antiinflammatory drugs, diuretics, laxatives) can contribute to AB-imbalances. The combination of mildly disturbed homeostasis and multimorbidity together with polypharmacy at an advanced age means that disturbances of AB-equilibrium are common. Despite the increase in vasopressin clearance, renal reactivity to it decreases with age, which in turn leads to reduced renal concentrating ability after water deprivation. In addition, in the elderly, the feeling of thirst is reduced, and thus, the drinking regime is affected by water deficit. The impaired concentration capacity of the kidneys and reduced thirst in old age can significantly increase the risk of dehydration during any serious illness.
Although insulin secretion may be impaired in old age, circulating insulin levels do not decrease, probably due to an age-related decrease in insulin clearance. The impaired glucose tolerance during old age is not generally the same as glycaemia in diabetics. According to the theory of aging, however, emphasizing protein glycation, even moderate long-term increases in glycaemia could influence the development of physiological deteriorations or diseases typically occurring in old age. In this regard, fasting blood glucose and glycated haemoglobin show a high degree of correlation in non-diabetics of older age in the broadest sense of the word. Some epidemiological studies indicate that increases in glycaemia can, therefore, accentuate the development of cardiovascular diseases even in non-diabetics. According to Sinclair, optimal fasting glycaemia in diabetics should be in the range of 7–9 mmol/l but should not exceed 10–13 mmol/l, even in very old and disabled diabetics. Even these disabled elderly diabetics should not be at risk of acute hypoglycaemia. DM affects up to 20% of people in the 7th decade, and another 20% suffer from impaired glucose tolerance. It is the most clinically significant metabolic disease of older age. In the case of seniors, it is mainly DM2T (up to 95% over 70 years).
Another endocrine variable of potential importance during aging is a growth hormone (insulin-like growth factor 1 – IGF 1). Pituitary secretion of the growth hormone decreases with age. Many of the anabolic effects of Growth Hormone (GH) are mediated just by IGF 1, which is produced in the liver and other tissues in response to GH. Similarly to how GH declines in old age, IGF 1 secretion declines with age as well. Changes in body composition in the elderly (i.e. muscle reduction and fat gain) are strikingly similar to findings in younger individuals with growth hormone deficiency. These findings led to a hypothesis that typical changes in body composition in old age are due to a decrease in growth hormone secretion.
During aging, the thyroid gland undergoes mild atrophy, fibrosis, ascent of colloid nodules and lymphocytic infiltration. In the senium, thyropathies will be detected in up to a tenth of individuals, more often in women. During the laboratory examination, there will often be asymptomatic forms, the so-called subclinical hypothyroidism and hyperthyroidism, with TSH deviation without an accompanying change in thyroxine (T4). Considering the initiation of potential therapy for both subclinical hypothyroidism and hyperthyroidism is a very controversial issue: the overall clinical picture and the degree of possible controllable symptoms from hypofunction or hyperfunction of the thyroid gland will be decisive. The prevalence of hypothyroidism increases significantly in old age. Its clinical symptomatology, however, is less noticeable than in younger individuals. Thyroxine (T4) secretion decreases by about 30% compared to young adults, while serum T4 levels remain unchanged. This decrease in T4 secretion is considered to be a physiological compensation for reduced tissue utilization and not a manifestation of primary thyropathy. The decrease in T4 utilization correlates with a decrease in the body’s active muscle mass. The Thyroid-Stimulating Hormone (TSH) level also increases slightly with age, which can probably explain the higher incidence of Hashimoto’s thyroiditis in old age. Autoimmune thyroiditis occurs in up to 1–2% of the elderly population, especially in women. A slight decrease in deiodination in the periphery is also compensatory in old age.
Ovaries show a dramatic decline in oestrogen and progesterone secretion as well as fibrosis and scarring as they get older. Menopause represents the most significant age-related endocrine syndrome. Menopause occurs on average around the age of 50 with accompanying hot flushes; the loss of bone mass and oestrogen-sensitive tissues is accentuated.
Andropause in men with a decrease in testosterone levels begins after the age of 50 (without affecting potency). Sexual functions are relatively well protected, although there is an increase in the refractory period and an increase in the time required for sexual arousal and loss of tissue turgor.
6. Multimorbidity: Characteristics
Multimorbidity (usually ≥3 diseases) represents the simultaneous presence of several diseases in one individual either without any causal relationship or with mutually causal conditioning. It brings along several risks not only for the sick seniors but also for their physicians. In essence, it belongs to the characteristics of the geriatric medicine [38].
Among the causes of morbidity in old age, diseases of the cardiovascular system conditioned by atherosclerosis (AS) occupy a leading place, such as coronary heart disease (CHD), heart- attack, angina pectoris, cerebrovascular events (stroke, TIA), ischaemic disease of the lower extremities – IDLE (apparent atherosclerosis – AS is present as an aetiology in up to 90% of people over 75 years of age) [48, 49]. The diseases of the locomotion system, sensory organs, tumours, injuries, diseases of the respiratory (chronic obstructive pulmonary disease – COPD), gastrointestinal (biliary and other problems) and urogenital system (prostate in men, gynaecological organs in women) are also common in old age [43]. Diabetes, and mental and nervous diseases are also frequent. The care for elderly people with multiple, mutually influencing diseases requires a great deal of diagnostic, analytical and synthetic skills and abilities of the physician, including his or her ability to deal with people [50]. Their current, often independent coexistence is exactly typical for senile multimorbidity. After the age of 60, diseases of the cardiovascular system (CHD, stroke, hypertension) show a steady rise, and the prevalence of DM also increases with age. The presence of the disease itself (or several diseases) is not decisive for the quality of life of a senior, but the degree of disability, i.e. functional impairment, to which it leads as a result. Full self-sufficiency can be maintained even in the presence of many diseases at the same time.
The interaction of different independent diseases in the elderly is a problem closely related to multimorbidity [5, 6, 9].
The objective effort to evaluate multimorbidity led to the creation of many internationally recognized scales (Kaplan-Feinstein Index; Charlson index); CIRS-G = Cumulative Illness Rating Scale – Geriatric Version; health-related quality of life (HRQOL); Disease Burden Morbidity Assessment (DBMA); Index of Co-Existent Disease (ICED); Geriatric Index of Comorbidity (GI); Functional Comorbidity Index (FCI); Total Illness Burden Index (TIBI).
The diseases often tend to accumulate and potentiate each other. Multidimensionality is typical in geriatric medicine. The seniors as a bio-psycho-social unit need to be understood much more significantly in old age than in younger age periods, both in the etiopathogenesis of diseases and in clinical practice. In geriatric medicine, psychological and social problems always appear simultaneously with somatic problems; both need to be solved just as urgently. This applies in the full sense of the word to completely typical primary somatic diseases characteristic of old age (strokes, degenerative diseases, tumours and immobilization). The same is fully true for diabetics in old age. Similarly, it is valid for the so-called “primarily” mental disorders (dementia, depression, delirium) or the so-called geriatric social syndromes (geriatric maladaptation syndrome, mistreatment, neglect and abuse).
Most clinicians tend to perceive multimorbidity as an unpleasant phenomenon associated with a decrease in functional capacities, cognitive impairment and, in addition to those, the risk of interactions between the diseases themselves and their possible pharmacotherapy. In the elderly (especially the advanced ones) multimorbidity is more the rule than the exception. The growth of the group of multimorbid seniors goes hand in hand with aging of the population in general. It has apparently increased in the last decade (by 22%). More than 75% of people aged ≥85 years are multimorbid.
6.1 Functional capacities: Multimorbidity and vice versa
Functional loss is the final state of most clinical problems in old age (especially late, i.e. over 80 years). This may be the only symptom of a serious ongoing disease, when the typical symptoms of the given disease are absent [33]. In practice, such functional damage means a decrease in the individual’s functional ability and can be easily assessed, for example, with the activities of daily living test (ADL score – assessment of these tasks: transfer, bed mobility, toileting and eating) and the instrumental test of daily activities (IADL – a tool used to determine the amount of help a person may need). In addition, it is also appropriate to assess cognition, nutritional, behavioural and social status in relation to the health status [51].
Older people, unlike younger people, react non-specifically to deterioration or illness, which actually means a decrease in some function. He or she stops eating and drinking, or new falls, confusion, lethargy, dizziness or incontinence occur. These symptoms may be the first or even the only manifestation of the disease, which in younger people has typical symptoms (such as pneumonia, heart attack, pulmonary embolism, myxedema, etc.). These functional deficits constitute geriatric syndromes (GS) [52]. These then significantly affect self-sufficiency without the presence of obvious or typical symptoms of any disease. In this regard, GS can be defined as a loss of functional capacities typically caused by multimorbidity, in which several organ systems are affected simultaneously.
Thanks to the rich range of medicines and progress in the pharmaceutical form technology, the physician can choose a medicinal product practically “tailored” to a specific men or women. On the other hand, however, this option brings along some negatives. This therapeutic effort can initially lead to a completely justified and purposeful pharmacotherapy, from which it is already very close to polypharmacy with possible health risks for the old people [53].
The vulnerability of the elderly through diseases in general is higher, and the balance of their organ homeostasis is quite fragile [54]. This is true for non-diabetic seniors and even more so for those being treated for diabetes and other related comorbidities. Similarly, it is true for the so-called primarily mental disorders (dementia, depression, delirium) or the so-called geriatric social syndromes (loneliness). Clinical picture of adaptation failure based on chronic stress in old age is a geriatric maladaptation syndrome, which can have a worse course in an elderly diabetic. The stressor is usually in the psychosocial area and its clinical manifestation occurs most frequently in the cardio – an cerebrovascular area (heart attack and stroke) or in the deterioration of immunity (bronchopneumonia), etc., with a higher risk of occurrence of complications in diabetics.
The mentioned somatic, psychological and social problems interact with each other and are characterized by difficult treatability, a chronic course with progression and a relatively unfavourable prognosis. They bring many difficult-to-manage situations and problems to the old people and their surroundings. In very old individuals (the so-called old-old, i.e. people over 80 years of age), diseases progress in a way that is usually significantly different from the course of diseases in middle age, and they also require a different approach, enabling the improvement of health status or at least the maintenance of existing self-sufficiency and accentuating the return to the home environment [55].
Pitfalls and negatives of multimorbidity represent:
Accelerated decline of the functional capacity
The higher incidence of symptoms and subjective complaints
The decline in quality of life
Increased mortality
Increased risk of hospitalization
Increased risk of institutionalization (nursing or residential home, etc.)
Rising health care costs
6.2 Atypical presentation of diseases in old age
Most biological functions (maximal respiratory capacity, glomerular filtration and cardiac index) reach their peak before the age of 30. Some of them then gradually decrease linearly [40, 44]. This decrease is practically insignificant for everyday normal activity, but it can become serious during periods of greater stress or under a load [56]. Physiological events that decrease with age are blood flow through the kidneys, creatinine clearance, maximum heart rate and, subsequently, stroke volume during exercises, glucose tolerance, vital lung capacity, body mass and cellular immunity [40, 44]. On the contrary, total lung capacity and liver function do not change relatively with age; ADH secretion even increases with age. Many of the above-mentioned declines, previously considered a natural consequence of aging, are significantly influenced by lifestyle, behaviour, diet and the environment in which the person has been living. The most important physiological change in old age is a predisposition to a higher incidence of serious diseases. The respiratory function of a healthy 70-year-old is about 50% of that of a 30-year-old one. Pneumonia is a common and severe disease in older people. In this group of men and women, pneumonia is among the four most frequent diseases leading to death. The diagnosis can often be difficult due to an atypical clinical presentation. Therefore, pneumonia should always be considered as the cause of any deterioration in an older person [57]. Geriatric problems, such as frailty, and physical and psychological limitations, should be recorded as well as the social situation [51]. Renal functions commonly decline by 50% or more after the 70th year of age. This decrease in physiological reserve capacity does not affect normal daily life but can complicate the ability to recover from a serious illness. At the same time, some physiological changes mimic a disease, even though they are a normal part of aging [6, 13]. An essential prognostic element in sepsis is the timeliness of acute treatment in elderly individuals with reduced immune defenses and physiological reserves [58]. Early control of comorbidities is the main plus value of the geriatrician in the acute and post-acute treatment of elderly people with sepsis [58]. In van Son study [59], men and women with an atypical presentation of COVID-19 were more frail compared to persons with a typical presentation. Contrary to our expectations, an atypical presentation was not associated with worse outcomes.
Diabetes mellitus (DM) can occur but also disappear in old age. The ability of insulin to stimulate glucose uptake declines with age and usually manifests itself as postprandial hyperglycaemia with constant normal fasting insulin and glucose levels. In stressful situations, diabetes can be detected in the elderly, but when the stressful situation is over, the chemical symptoms of DM also disappear. This loss of physiological reserves understandably contributes to the increasing prevalence of DM with increasing age. The age-related changes that make older people more vulnerable in everyday life tend to be subtle [60]. In the elderly, both hypothermia and hyperthermia can develop more easily during exposure to extreme environmental influences, as there are several changes in the management of thermoregulation, including those in the field of neurology. The loss of brainstem neurotransmitters can not only cause the typical old-age gait but also predisposes genetically determined individuals to the development of, e.g. Parkinson’s disease. Some age-related changes cause specific consequences. Menopause is a physiological event associated with normal aging, but it leads to symptoms that predispose the body to a bone loss and atherosclerosis.
Apart from clinically manifest forms of diseases, a subclinical form of diseases is common in the elderly. Among 6000 individuals over the age of 65 participating in the Cardiovascular Health Study, 31% had clinically manifest cardiovascular disease, while another 37% had a subclinical form of the disease detected by non-invasive examinations.
Atypical or symptom-poor symptomatology of diseases is even higher in elderly diabetics than in the general population. This makes them very risky both in terms of diagnosis and possible therapy. The whole problem is greatly complicated by the decline of physiological functions and changes in organ systems in old age, which can often (and in diabetics) border on pathology more significantly.
Commonly present symptoms typical of a certain diagnosis are missing
On the contrary, changes in behaviour and functional abilities are present
Failure to make a correct diagnosis with atypical symptomatology can lead to wrong conclusions, wrong diagnosis and wrong treatment
Seniors come to central admissions for chest pains lasting more than 6 hours, although they were cardiac people with proven CHD. Among the elderly (70–80 years) and very old (>80 years) subjects who had an acute myocardial infarction, in-hospital mortality was two to three times higher than in younger subjects (<70 years). It emphasizes the need to improve education among older people in relation to some symptoms. Some symptoms such as a loss of consciousness or paralysis of the limbs are understandably never considered normal. Although some symptoms of psychiatric illnesses were not considered normal, they were rarely attributed to serious illnesses (e.g. dementia, etc.).
Although the oldest people had the best prospects for good health, they were also the least able to respond to symptoms of illness. The very old seniors most frequently attributed the symptoms to aging itself and reacted to them by: (1) waiting and watching; (2) by accepting; (3) by denying the danger; (4) postponement or refused medical care. Although the underestimation of disease symptoms in old age with dangerous associations is well-known, many clinicians have in mind the image of the elderly with endless longings, whose investigation, in the end, leads nowhere. The risk of underestimation of symptoms in old age is obvious. Late recognition of the disease leads to delayed initiation of therapy, usually after a more severe course of the disease, which leads to serious consequences and causes deeper functional impairment. Rehabilitation to independence tends to be difficult in these cases, and sometimes, despite “successful treatment,” permanent addiction can result. Therefore, the message to clinicians is clear: early detection and therapy of sick people and early detection of at-risk seniors must be the priority.
The pitfalls and negatives of multimorbidity are:
Accelerated decline of functional capacities
Higher occurrence of symptoms and subjective problems
Decline of life quality
Higher mortality
Higher risk of hospitalization
Higher risk of placement in institutional care (home for the elderly, etc.)
Rising health care costs
According to the data published by Medicare [61], the rise in the costs of care per person and year is quite obvious: from $211 for people without a chronic diagnosis (chr.dg.); to those with ≥2 chr. dg. – $1870; ≥ 5 chr. dg. – $8159 and ≥ 7 chr. dg. – $23,000.
Gathering basic information requires the physician’s patience, time and information from the family and care providers.
The first symptoms of an impending infection or serious illness across the disease spectrum can be completely inconspicuous in the elderly. They can present themselves as:
Change of function: new incontinence; new disturbances in standing or walking;
Behavioural changes: agitation; delirium;
Other indicators of change in functional potential: falls, anorexia, fatigue, and weakness.
Due to homeostenosis and a decrease in functional reserves, clinical manifestations may appear in some cases, even at an early stage. Here, even a small improvement of specific symptoms can significantly improve the overall condition of the senior (self-sufficiency). E.g., heart failure can be precipitated by mild hyperthyroidism, a urinary retention by beginning benign prostatic hyperplasia, and hyperosmolar non-ketoacidotic coma in mild glucose intolerance. Paradoxically, the underlying disease can be treated at this stage easily because it is not advanced. Unfortunately, in old age, the development of clinical symptoms (including the ability to describe them) may be limited by reduced mobility and cognitive impairment. For these very reasons, CHD or aortic stenosis can be asymptomatic for a certain period of time.
7. Polypharmacy and old age in combination with multimorbidity
There is a lack of evidence for the specific treatment of multimorbid seniors, as they are usually excluded from large randomized clinical trials (RCT). Exclusion from trials of older people and people with comorbidity and co-prescribing is increasingly untenable given the population ageing and increasing multimorbidity [62]. In a retrospective analysis of five general medical periodicals with the highest IF (impact factor) – out of 284 RCTs from 1995 to 2010, 65% of seniors were excluded due to multimorbidity. In an evaluation of 11 Cochrane Review RCTs according to the presence of four typical chronic diseases (diabetes, heart failure, COPD and stroke), less than half of the participants in these studies had some of the listed chronic diagnoses when they met the entry criteria. Multimorbid seniors usually are not included in RCTs at all. Clinical guidelines usually do not consider multimorbidity at all (or minimally) and often do not even provide any recommendations that would take into account other comorbidities occurring at the same time, which fully can be applied to older diabetics as well. Despite the great individual and societal burden associated with multimorbidity, little is known about how to effectively manage it [63].
A complex health and social situation is common in multimorbidity. It can often lead to complicated treatment regimens that are difficult for seniors to understand and demanding for physicians to explain. The attending physician requires the ability to coordinate the therapy of various organ systems with other physicians (specialists of various medical branches, including subfields of internal medicine) and the skill of communication with elderly people and their families, so that therapeutic efforts are not only correctly understood, but also carried out. In geriatrics, the primary problem is precisely the lack of studies that would include precisely these multimorbid seniors. This deficit is generally known.
Evidence-based medicines (EBMs) are based on RCTs conducted on defined populations where inclusion and exclusion criteria are applied. Physicians must apply them in the specific situations of individual people. The situation is relatively simple if your men or women belong to the group of fit seniors who meet the guidelines’ criteria. It becomes very difficult if they belong to typical geriatric multimorbid persons who are frail and multimorbid and have polypharmacy, cognitive impairment or other functional deficits [64]. Physicians acting strictly according to the guidelines can run into certain risks. Regimens can be either too general or demanding and expensive. An individual approach to the elderly is necessary for choosing a treatment strategy and interventions in general. It is crucial to strictly follow the START/STOP recommendations [65]. A hypothetical patient with hypertension, COPD, DM2, osteoporosis and osteoarthritis should take up to 12 different medications per day according to the guidelines.
Physicians in general are led to use EBM as the gold standard in daily clinical practice. Unfortunately, most RCTs work only with ideal persons, where seniors with a greater risk of developing adverse drug reactions (ADRs), advanced disease and a possible risk of not completing the study are excluded. These non-ideal multimorbid persons, often subject to exclusion criteria, are precisely the typical elderly people of our geriatric practice.
The question the physician should ask before applying any standards and templates is whether and how our men or women are different from the RCT one. It is important to note that the guidelines can usually be applied to relatively healthy seniors. ADRs can exacerbate some of the comorbidities. In patients with functional limitations and disorders, there will be justified fears of falls (antihypertensive therapy, etc.) [66]. The use of warfarin and similar new anticoagulants (dabigatran, rivaroxaban, apixaban, etc.) requires the cooperation of the patient (or a caregiver). Their effective use can be limited in the elderly by possible cognitive impairment (close control of blood clotting - International Normalized Ratio (INR). RCTs focus on outcomes such as mortality or organ-specific outcomes (e.g. cardiovascular endpoints). Other outputs that are often essential in geriatrics for seniors such as maintaining self-sufficiency, mobility and fall prevention or quality of life are often not included in the studies.
7.1 Is there any effective polypharmacy in multimorbid seniors?
Nowadays, thanks to a very wide range of medicines and progress in the field of pharmaceutical form technology, a physician can choose a medicine practically “tailored” to a specific senior [67]. On the other hand, this option brings some pitfalls [68]. Above all, it puts high demands on the professional competence of the physician, on his or her orientation in available medicines and on detailed knowledge of their possibilities and potential risks. For physicians in ambulatory practice, the results of recent studies are sometimes problematically available. This therapeutic effort can initially lead to a completely justified and expedient pharmacotherapy, from which it is already very close to polypharmacy with possible health risks for multimorbid seniors, regardless of the decreasing compliance and increasing costs of therapy [68]. The elderly people living in institutional care consume three times more drugs compared to the same number of individuals in the entire population, and women twice as many as compared to men.
Problems accompanying pharmacotherapy in old age are:
High economic costs of care and the need to consider the benefit of therapy for the patient’s prognosis.
High degree of polypharmacotherapy and multimorbidity with frequent medication complications.
Inadequate diagnosis, treatment of some diseases and, more than once, underestimation of some symptoms (pain in old age, depression, etc.)
Low compliance. In old age, non-compliance is present in 30–50%.
7.2 A basic requirement for pharmacotherapy in old age
Simplicity, expediency and efficiency are essential. Polypragmasy in older age can often be risky and ineffective, many times even harmful. In geriatric medicine, symptomatic treatment generally prevails over the causal treatment. Its adverse effects can fundamentally alter the clinical picture of diseases.
The elderly are generally more vulnerable, the therapeutic range becomes narrow, the compliance decreases, interindividual variability of the effect increases, and the risk of drug interactions increases [69]. In old age, there is a rise in gastric pH, a decrease in gastric and intestinal motility and a decrease in GIT blood flow. Despite these changes, the absorption of most drugs is not significantly affected in old age. Among the most frequent and clinically significant drug interactions are those at the biotransformation level. Inducers and inhibitors of metabolism through the modulation of hepatocyte microsomal enzymes (first pass effect cytochrome CYP 450) substantially affect the levels of other drugs biotransformed by these enzymes. The bioavailability of a drug is determined by the amount of drug that enters the systemic circulation. Thus, it is significantly dependent on gastrointestinal absorption and first pass through the liver.
In older age, most people experience significant changes in the effects of many medications [70]. The spectrum of drugs used changes with age and generally has an upward trend towards polypharmacotherapy. Multimorbidity, nutritional problems and an increased risk of non-compliance are typical. These facts, combined with changes in pharmacokinetics and pharmacodynamics, can lead to serious adverse side effects and drug interactions. With age, the interindividual variability of the doses required to achieve a therapeutic effect increases in general. Pharmacotherapy in geriatric medicine can have significant potential not only therapeutic but also toxic.
Reducing excessive polypharmacy means benefiting the patient’s health, improving adherence to treatment and reducing drug costs. Scott [66] directly states that no study has shown that changes (reductions) in polypharmacy could reduce morbidity or mortality in the elderly but lead to a decrease in ADRs (by 35%), a decrease in costs and an improvement in compliance with the remaining medication. De-prescribing is an important concept in older people, given the harm associated with polypharmacy in this age group [71, 72]. Holmes [69] emphasizes geriatric prescription on defining health care goals, individual therapeutic goals, expected average life expectancy and chance of survival, and the need to start with a smaller dose and slowly increase it to the effect. The health care goals in general for the elderly can vary from curative to purely palliative care. Similarly, therapeutic goals may range from preventive to immediate curative [73].
8. Conclusion
As an interdisciplinary field, geriatric medicine emphasizes a holistic approach and the need to perceive older individuals from the point of view of bio-psycho-social integrity, while emphasizing maintaining or improving self-sufficiency and independence for as long as possible. In the broadest sense of the word, successful aging in good physical and mental condition with an acceptable degree of independence and possible active engagement is the de facto goal of modern clinical gerontology.
The increase in average life expectancy (LE) brings along a whole range of health and social problems, not only for the seniors themselves but also for their families and society. The aging of the population, as a typical phenomenon of modern society, requires a change of thinking and attitudes in many areas, starting with the conservatism of doctors and ending with the inattention of politicians. Life expectancy comparisons (see Figure 7) is significantly influenced by the composition of the population and will be applied even more significantly in the coming decades (in the Czech Republic is LE 77.2 y., for men 74.1 and women 80.5 in 2020).
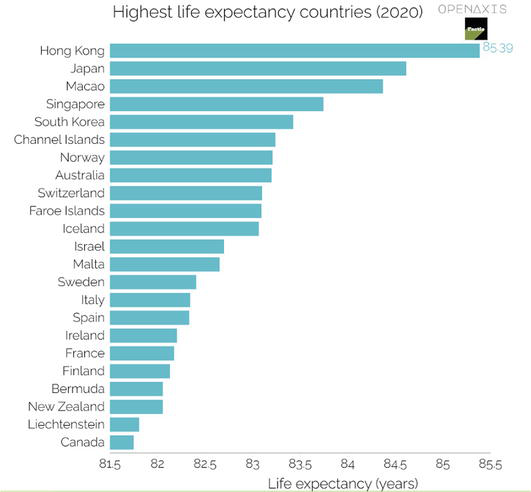
Figure 7.
Highest life expectancy country in 2020 (according Openaxis).
When choosing DM therapy, the following should be taken into account: the age of the diabetic, including expected life expectancy; the presence of macro- and microangiopathic complications; degree of self-sufficiency, family environment and economic conditions; catering habits (including state of teeth); other handicaps – mental and motor, sight and hearing.
Therefore, the main goal of DM treatment in old age is to improve the quality of life. It can be summarized in the following few points:
optimal metabolic compensation
prevention of the following symptoms: fatigue, weight loss, polyuria, infectious complications
definition of lifestyle requirements, especially dietary habits
limitation of drugs used – especially with the risks of hypo- and hyperglycaemia
If insulin therapy is initiated by the clinician, the following questions should always be answered:
who will administer the insulin?
what are the checking options (including glycaemia)?
who will help the diabetic during contingent hypoglycaemia if he lives alone, and how?
References
- 1.
Comer A, Fettig L, Torke AM. Identifying goals of care. The Medical Clinics of North America. 2020; 104 (5):767-775 - 2.
Hariharan R, Odjidja EN, Scott D, et al. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obesity Reviews. 2022; 23 (1):e13349 - 3.
Edelman SV, Henry RR. Diagnosis and Management of Type 2 Diabetes. 13th ed. New York: Professional Communications Inc.; 2017. p. 528 - 4.
Sinclair AJ, Dunning T, Manas LR, Munshi M. Diabetes in Old Age. 4th ed, Kindle ed. Hoboken, New Jersey: Wiley-Blackwell; 2017. p. 557 - 5.
Halter J, Ouslander J, Studenski S, High K, Asthana S, Supiano M, et al. Hazzard’s Geriatric Medicine and Gerontology. 8th ed. NY: McGraw Hill/Medical; 2022. p. 1824 - 6.
Morley JE, Vellas B, Sinclair AJ, Cesari M, Munshi M. Pathy’s Principles and Practice of Geriatric Medicine. 6th ed. Hoboken, New Jersey: Wiley-Blackwell; 2022. p. 1856 - 7.
Smailhodzic E, Hooijsma W, Boonstra A, Langley DJ. Social media use in healthcare: A systematic review of effects on patients and on their relationship with healthcare professionals. BMC Health Services Research. 2016; 16 :442. Article number: 442. DOI: 10.1186/s12913-016-1691-0 - 8.
Lesley KB, James DP, Kunal S, et al. Oxford Handbook of Geriatric Medicine. Third ed. UK: Oxford University Press; 2019 - 9.
Fillit HM, Rockwood K, Young JB. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. Philadelphia: Elsevier Health Sciences; 2016. p. 1168 - 10.
Balsa A, Diáz C. Social interactions in health behaviors and conditions. Published online: 26 March 2019. DOI: 10.1093/acrefore/9780190625979.013.17 - 11.
Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: Implications for clinical practice and public health. Lancet. 2019; 394 (10206):1365-1375 - 12.
Weber P. Ageing of old community (seniorization) and geriatrization of medicine (in Czech). Geri a Gero. 2018; 7 (4):152-155 - 13.
Krajčík Š, a kol. (Ed. in Slovac and Czech): Geriatria, 2. vydání, doplněné a přepracované. Bratislava: Herba; 2022. p. 584 - 14.
Ritt M, Ritt JI, Sieber CC, Gaßmann KG. Comparing the predictive accuracy of frailty, comorbidity, and disability for mortality: A 1-year follow-up in patients hospitalized in geriatric wards. Clinical Interventions in Aging. 2017; 12 :293-304 - 15.
Romero-Ortuno R, Wallis SJ, Biram RW, Keevil V. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults: An observational study. European Journal of Internal Medicine. 2016; 35 :24-34 - 16.
Weber P. Elderly Patients with Multi-Morbidity, Frailty and Geriatric Syndromes: Therapeutic Problem of Contemporary and Incoming Medicine at Intensive Care Unit (ICU) from the Geriatricians Viewpoint. New York: Nova Science Publishers; 2011. p. 82 - 17.
Fried LP, Cohen AA, Xue QL, et al. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nature Aging. 2021; 1 (1):36-46 - 18.
Kastner M, Cardoso R, Lai Y, Treister V, Hamid JS, Hayden L, et al. Effectiveness of interventions for managing multiple high-burden chronic diseases in older adults: A systematic review and meta-analysis. CMAJ. 2018; 190 (34):E1004-E1012 - 19.
Ghachem A, Fried LP, Legault V, Bandeen-Roche K, Presse N, Gaudreau P, et al. Evidence from two cohorts for the frailty syndrome as an emergent state of parallel dysregulation in multiple physiological systems. Biogerontology. 2021; 22 (1):63-79 - 20.
Laurindo LF, Barbalho SM, Guiguer EL, et al. GLP-1a: Going beyond traditional use. International Journal of Molecular Sciences. 2022; 23 (2):739 - 21.
Saito T, Watanabe M, Nishida J, et al. Zensharen study for prevention of lifestyle diseases group. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: A randomized controlled trial. Archives of Internal Medicine. 2011; 171 (15):1352-1360 - 22.
Billings LK, Parkin CG, Price D. Baseline glycated Hemoglobin values predict the magnitude of Glycemic improvement in patients with type 1 and type 2 diabetes: Subgroup analyses from the DIAMOND study program. Diabetes Technology & Therapeutics. 2018; 20 (8):561-565 - 23.
American Diabetes Association. 9. Pharmacologic approaches to Glycemic treatment: Standards of medical Care in Diabetes-2021. Diabetes Care. 2021; 44 (Suppl.1):S111-S124 - 24.
Weber P, Meluzínová H, Weberová D. New trends in the approach to the treatment of type 2 diabetes – Observations and benefits in the outpatient practice of a diabetologist (in Czech). Klinická Farmakologie a Farmacie. 2021; 35 (4):3-8 - 25.
Weber P, Meluzínová H, Weberová D. Trajectories of changes I – in the approach to oral hypoglycemic therapy of type 2 diabetes in post-productive age during the past 12 years – Our own experience (in Czech). Geri a Gero. 2020; 9 (3):61-65 - 26.
Sinclair AJ, Paolisso G, Castro M, et al. European diabetes working party for older people. European diabetes working party for older people 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes & Metabolism. 2011; 37 (Suppl. 3):S27-S38 - 27.
Bramlage P, Gitt AK, Binz C, Krekler M, et al. Oral antidiabetic treatment in type-2 diabetes in the elderly: Balancing the need for glucose control and the risk of hypoglycemia. Cardiovascular Diabetology. 2012; 6 (10):111-122 - 28.
Fayfman M, Galindo RJ, Rubin DJ, et al. A randomized controlled trial on the safety and efficacy of exenatide therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes. Diabetes Care. 2019; 42 (3):450-456 - 29.
Chon S, Rhee SY, Ahn KJ, et al. KIIT study investigators. Long-term effects on glycaemic control and β-cell preservation of early intensive treatment in patients with newly diagnosed type 2 diabetes: A multicentre randomized trial. Diabetes, Obesity & Metabolism. 2018; 20 (5):1121-1130 - 30.
Mahaffey KW, Neal B, Perkovic V, et al. CANVAS program collaborative group. Canagliflozin for primary and secondary prevention of cardiovascular events: Results from the CANVAS program (Canagliflozin cardiovascular assessment study). Circulation. 2018; 137 (4):323-334 - 31.
Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019; 139 (17):2022-2031 - 32.
Charytan DM, Solomon SD, Ivanovich P, et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes, Obesity & Metabolism. 2019; 21 (5):1199-1208 - 33.
Mendes R, Sousa N, Themudo-Barata JL, Reis VM. High-intensity interval training versus moderate-intensity continuous training in middle-aged and older patients with type 2 diabetes: A randomized controlled crossover trial of the acute effects of treadmill walking on Glycemic control. International Journal of Environmental Research and Public Health. 2019; 16 (21):4163 - 34.
Rossello X, Ferreira JP, McMurray JJ, et al. Editor’s choice-impact of insulin-treated diabetes on cardiovascular outcomes following high-risk myocardial infarction. European Heart Journal Acute Cardiovascular Care. 2019; 8 (3):231-241 - 35.
Yang Y, Xinhua H, Zhang Q , Zou R. Diabetes mellitus and risk of falls in older adults: A systematic review and meta-analysis. Age and Ageing. 2016; 45 (6):761-767 - 36.
Weber P, Weberová D, Matějovská Kubešová H, Meluzínová H, Jarkovský J, Bieláková K, et al. Falls in anaemic hospitalized elderly patients in 2012-2016–Mutual relationships. Advances in Gerontology. 2019; 32 (5):787-794 - 37.
Nooijen CFJ, Blom V, Ekblom O, et al. The effectiveness of multi-component interventions targeting physical activity or sedentary behaviour amongst office workers: A three-arm cluster randomised controlled trial. BMC Public Health. 2020; 20 (1):1329 - 38.
Zulman DM, Sussman JB, Chen X, Cigolle CT, Blaum CS, Hayward RA. Examining the evidence: A systematic review of the inclusion and analysis of older adults in randomized controlled trials. Journal of General Internal Medicine. 2011; 26 (7):783-790 - 39.
Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson JL. Harrison’s Principles of Internal Medicine, Twenty-First Edition. 21st ed. Vol. 1 & 2. NY Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto: McGraw Hill/Medical; 2022. p. 4384 - 40.
Barrett K, Barman S, Yuan J, Brooks H. Ganong’s Review of Medical Physiology. 26th ed. NY: McGraw Hill/Medical; 2019. p. 752 - 41.
Nascimento CM, Ingles M, Salvador-Pascual A, et al. Sarcopenia, frailty and their prevention by exercise. Free Radical Biology & Medicine. 2019; 132 :42-49 - 42.
Cederholm T, Morley JE. Sarcopenia: The new definitions. Current Opinion in Clinical Nutrition and Metabolic Care. 2015; 18 (1):1-4 - 43.
Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. Journal of the American Medical Association. 2005; 294 (6):716-724 - 44.
Dansereau G, Wey TW, Legault V, et al. Conservation of physiological dysregulation signatures of aging across primates. Aging Cell. 2019; 18 (2):e12925 - 45.
Motta M, Bennati E, Ferlito L, Malaguarnera M. Diabetes mellitus in the elderly: Diagnostic features. Archives of Gerontology and Geriatrics. 2006; 42 :101-106 - 46.
Huang ES, Laiteerapong N, Liu JY, et al. Rates of complications and mortality in older patients with diabetes mellitus: The diabetes and aging study. JAMA Internal Medicine. 2014; 174 (2):251-258 - 47.
Ni Lochlainn M, Cox NJ, Wilson T. Nutrition and frailty: Opportunities for prevention and treatment. Nutrients. 2021; 13 (7):2349 - 48.
Azad N, Molnar F, Byszewski A. Lessons learned from a multidisciplinary heart failure clinic for older women: A randomised controlled trial. Age and Ageing. 2008; 37 (3):282-287 - 49.
Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: A study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998; 98 (21):2282-2289 - 50.
American Geriatrics Society expert panel on the Care of Older Adults with multimorbidity. Guiding principles for the care of older adults with multimorbidity: An approach for clinicians. Journal of the American Geriatrics Society. 2012; 60 (10):E1-E25 - 51.
Rodríguez-Romero R, Herranz-Rodríguez C, Kostov B, et al. Intervention to reduce perceived loneliness in community-dwelling older people. Scandinavian Journal of Caring Sciences. 2021; 35 (2):366-374 - 52.
Inouye SK. Geriatric syndromes: Clinical, research, and policy implications of a Core geriatric concept. Journal of the American Geriatrics Society. 2007; 55 (5):780-791 - 53.
Expert Panel. American Geriatrics Society updated beers criteria for potentially inappropriate medication use in older adults. American Geriatrics Society 2012 beers criteria update. Journal of the American Geriatrics Society. 2012; 60 (4):616-631 - 54.
Jadad AR, Matthew JT, Emara M, Jones J. Consideration of multiple chronic diseases in randomized controlled trials. Journal of the American Medical Association. 2011; 306 (24):2670-2672 - 55.
Wein B, Seleznova Z, Mueller D, Naumann M, Loeser S, Artmann J, et al. Evaluation of the guideline-adherence of coronary angiography in patients with suspected chronic coronary syndrome - results from the German prospective multicentre ENLIGHT-KHK project. International Journal of Cardiology. Heart & Vasculature. 2023; 46 :101203. DOI: 10.1016/j.ijcha.2023.101203 eCollection 2023 Jun - 56.
Emmett KR. Nonspecific and atypical presentation of disease in the older patient. Geriatrics. 1998; 53 (2):50-52 - 57.
Frohnhofen H, Stieglitz S. Lungenentzündung im Alter. Pneumologe (Berl). 2021; 18 (3):174-181 - 58.
Putot A, Prendki V. New horizons in sepsis management in older patients. Age and Ageing. 2023; 52 (2):afad016. DOI: 10.1093/ageing/afad016 - 59.
van Son JE, Kahn ECP, van der Bol JM, Barten DG, Blomaard LC, van Dam C, et al. Atypical presentation of COVID-19 in older patients is associated with frailty but not with adverse outcomes. European Geriatric Medicine. 2023; 14 (2):333-343 - 60.
Zhao Q , Yin K, Zhou N, Wu Q , Xiao Y, Zheng J, et al. The characteristics of thoracic aortic dissection in autopsy-diagnosed individuals: An autopsy study. Frontiers in Cardiovascular Medicine. 2022; 9 :973530. DOI: 10.3389/fcvm.2022.973530 eCollection 2022 - 61.
Boult C, Wieland GD. Comprehensive primary care for older patients with multiple chronic conditions: “Nobody rushes you through”. Journal of the American Medical Association. 2010; 304 (17):1936-1943 - 62.
He J, Morales DR, Guthrie B. Exclusion rates in randomized controlled trials of treatments for physical conditions: A systematic review. 2020; 21 (1):228 - 63.
Skou ST, Nyberg M, Dideriksen M, Overgaard JA, Bodilsen C, Soja AM, et al. Study protocol for a multicenter randomized controlled trial of personalized exercise therapy and self-management support for people with multimorbidity: The MOBILIZE study. Journal of Multimorbidity & Comorbidity. 2023; 13 :26335565231154447. DOI: 0.1177/26335565231154447 eCollection 2023 Jan-Dec - 64.
Eidam A, Roth A, Lacroix A, Goisser S, Seidling HM, Haefeli WE, et al. Methods to assess patient preferences in old age pharmacotherapy - a systematic review. Patient Preference and Adherence. 2020; 14 :467-497 - 65.
Gallagher P, O’Mahony D. STOPP (screening tool of older Persons’ potentially inappropriate prescriptions): Application to acutely ill elderly patients and comparison with Beers’ criteria. Age and Ageing. 2008; 37 (6):673-679 - 66.
Cott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: A 10-step conceptual framework. The American Journal of Medicine. 2012; 125 (6):529-537 - 67.
Gurwitz JH. Polypharmacy: A new paradigm for quality drug therapy in the elderly? Archives of Internal Medicine. 2004; 164 (18):1957-1959 - 68.
Steinman MA, Handler SM, Gurwitz JH, Schiff GD, Covinsky KE. Beyond the prescription: Medication monitoring and adverse drug events in older adults. Journal of the American Geriatrics Society. 2011; 59 (8):1513-1520 - 69.
Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Archives of Internal Medicine. 2006; 166 (6):605-609 - 70.
Aubert CE, Blum MR, Gastens V, Dalleur O, Vaillant F, Jennings E, et al. Prescribing, deprescribing and potential adverse effects of proton pump inhibitors in older patients with multimorbidity: An observational study. CMAJ Open. 2023; 11 (1):E170-E178. DOI: 10.9778/cmajo.20210240. Print 2023 Jan-Feb - 71.
Reeve J, Maden M, Hill R, Turk A, Mahtani K, Wong G, et al. Deprescribing medicines in older people living with multimorbidity and polypharmacy: The TAILOR evidence synthesis. Health Technology Assessment. 2022; 26 (32):1-148. DOI: 10.3310/AAFO2475 - 72.
Seewoodharry M, Khunti K, Davies MJ, Gillies C, Seidu S. Attitudes of older adults and their carers towards de-prescribing: A systematic review. Diabetic Medicine. 2022; 39 (7):e14801. DOI: 10.1111/dme.14801 Epub 2022 Feb 15 - 73.
Reeve E, Low L-F, Hilmer SN. Attitudes of older adults and caregivers in Australia toward deprescribing. Journal of the American Geriatrics Society. 2019; 67 (6):1204-1210