Abstract
In this chapter, we will address the effectiveness of these two powerful insecticidal plant species on the survival of eggs and larvae of Bemisia spp. (Hemiptera), Sordidus spp. (Coleoptera) and Spodoptera spp. (Lepidoptera). To obtain the essential oil, the cold pressing method described by Pinheiro and adapted by Barroso was used, where 900 g of seeds of the plant material was placed in the oven at a temperature of 45 ± 2°C for 48 h, then the seeds were crushed in a Britânia Diamante Black 4 blender at a speed of 900 W. It was pressed manually in an oil press machine. The resulting aqueous material was placed in a container and sealed with parafilm to prevent evaporation. It was left to rest in the dark at a temperature of 18 ± 2°C in an oven for 48 h. It was decanted to separate the essential oil and then filtered to remove suspended solid particles. For the Bioensio with eggs and larvae, four flasks covered with fine mesh were used, each containing 50 eggs of each species, at a temperature of 25 ± 2°C. For both cases, 2 ml of essential oil from the three prepared solutions was used. It was verified that: the essential oils of Ricinus communis are effective on the mortality of Bemisia spp., Spodoptera spp. and Sordidus spp. larvae, reaching 100% mortality in 7, 9 and 9 days of exposure, respectively; the essential oils of Azadirachta indica are effective on the mortality of Bemisiaspp., Spodoptera spp. and Sordidus spp. larvae, reaching 100% mortality in 9, 7 and 6 days of exposure, respectively; the solution of Azadirachta indica and Ricinus communis was the most effective in achieving mortality on Bemissia spp., Spodoptera spp. and Sordidus spp. in 5, 4 and 5 days, respectively.
Keywords
- plants
- Azadirachta indica
- Ricinus communis
- mortality
- insects
1. Introduction
Chemical warfare is a problem that has been going on for many decades. The effects of the use of chemical insecticides led Carson [1] to describe in his work Silent Spring the great environmental consequences resulting from the use of these substances.
For a long time, dichloro-diphenyl-trichloroethane (DDT) was used, but this compound has the ability to persist for a long time in the environment, accumulating in animal and vegetable organisms, thus its use was disapproved. However, the frequent use of insecticides (such as: organophosphates, carbamates and pyrethroids) can lead to the development of insect resistance to these compounds, compromising control and favoring the transmission of diseases [2]. In addition to the development of population resistance to insecticides, there may be a decrease in the population of natural enemies, health risks for humans and animals, contamination of groundwater and a decrease in biodiversity [3].
The environmental problems arising from the use of these chemicals, including public health, led researchers, scientists and others to direct the fight against vectors to another dimension, thinking about sustainability. As a result, background knowledge on phytopharmaceuticals used in some regions of the world for decades was used. For a long time, plants served as a medicinal base for human civilizations.
Therefore, the use of plants with insecticidal properties is not a recent practice. The first phytoinsecticides were pyrethrin extracted from chrysanthemum
In this chapter, we want to evaluate the insecticidal effect of two plant species with great potential,
Researchers have discovered that neem works both in the pesticide and medicinal areas. Its seeds and leaves have been found to combat more than 200 species of insects, cockroach pests, moths, aphids, among others. The tree is probably the only and best source of biopesticide in existence, a potential plant.
There are studies conducted by researchers on the effect of castor on insects, for example, Barroso [5, 6] evaluated the effect of this plant on
Specifically, we will address the effectiveness of these two powerful insecticidal plant species on the survival of eggs and larvae of
2. Methodology
2.1 Collection and identification of plant material
For the bioactivity tests on

Figure 1.
Leaves and fruits of

Figure 2.
Leaves and fruits of
2.2 Obtaining essential oil from Azadirachta indica and Ricinus communis seeds
To obtain the essential oil, the cold pressing method described by Pinheiro (2003) and adapted by Barroso [5, 6] was used, where 900 g of seeds of plant material was placed in the oven at a temperature of 45 ± 2°C for 48 h, then the seeds were crushed in a Britânia Diamante Black 4 blender at a speed of 900 W.
It was pressed manually in an oil press machine. The resulting aqueous material was placed in a container and sealed with parafilm to prevent evaporation. It was allowed to stand in the dark at a temperature of 18 ± 2°C in an oven for 48 h. Subsequently, a heterogeneous compound was obtained with a light phase rich in oil on the surface, an intermediate phase rich in water and a heavy phase rich in insoluble solids. It was decanted to separate the essential oil and then filtered to remove suspended solid particles.
2.3 Bioassay
For the Bioensio with eggs, four flasks covered with fine mesh were used, each containing 50 eggs of each species, at a temperature of 25 ± 2°C. For the bioassay with larvae, four flasks were used, each containing 50 eggs, waiting for hatching, where the larvae were obtained. For both cases, 2 ml of

Figure 3.
Schematic illustration of the experimental design of the bioassay used for the essential oils of the two species of plants:
3. Results and discussions
3.1 Effect on egg hatching rate
The analysis of the figure below demonstrates a weak correlation between the essential oils of

Figure 4.
Graphic illustration of the effect of
Similarly, a weak correlation similarity was observed between the essential oils of

Figure 5.
Graphic illustration of the effect of
It was observed that 5% of the population began to occlude on the 6th day of exposure. However, there were still no significant changes since, like the experiments described above, the joint action does not influence the occlusion rates of

Figure 6.
Graphic illustration of the effect of the joint action of the essential oils of
Regarding the effect of

Figure 7.
Graphic illustration of
The effect of

Figure 8.
Graphic illustration of
The effect of the joint action of essential oils on

Figure 9.
Graphic illustration of the effect of the joint action of the essential oils of
The effect of

Figure 10.
Graphic illustration of
The effect of

Figure 11.
Graphic illustration of
The effect of the joint action of essential oils on

Figure 12.
Graphic illustration of the effect of the joint action of the essential oils of
3.2 Effect on larval mortality
Regarding the effect of

Figure 13.
Graphic illustration of the effect of the essential oil of
Regarding the effect of

Figure 14.
Graphic illustration of the effect of
The combined action demonstrates a similar effect as the isolated action of

Figure 15.
Graphic illustration of the effect of the joint action of
The effect of
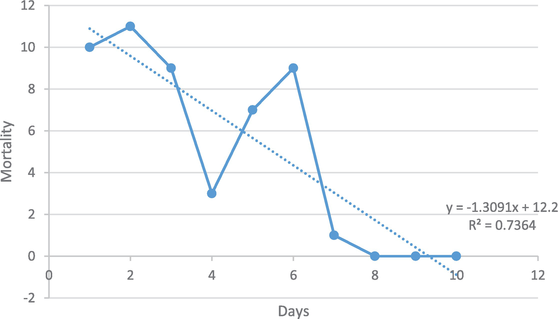
Figure 16.
Graphic illustration of the effect of
Similar to Figure 10, it was observed that the effect of

Figure 17.
Graphic illustration of the effect of the essential oil of
The joint action proved to be very efficient on

Figure 18.
Graphic illustration of the effect of the joint action of the essential oils of
The effect of

Figure 19.
Graphic illustration of the effect of
Similar to previous

Figure 20.
Graphic illustration of the effect of
The joint action proved to be very efficient on

Figure 21.
Graphic illustration of the effect of the joint action of
4. Discussion
The effects of these plants have already been tested by other authors, like Peron and Ferreira [8], who evaluated the efficiency of
At higher concentrations of
The effect of
The efficacy of
Several studies have been carried out in recent years to elucidate the changes in the endocrine control mechanism induced by azadirachtin, which cause the effects observed in growth inhibition. These studies made it possible to identify changes in the levels of morphogenetic hormones such as ecdysone [14].
A marked structural similarity was identified between ecdysone and azadirachtin; however, it is not clear whether the effects on these hormonal levels are direct or indirect [14].
Some evidence indicates that azadirachtin can block the release of several substances located in the central nervous system, as well as the formation of chitin, a polysaccharide that forms the exoskeleton of insects, in addition to preventing sexual communication, causing sterility and decreasing intestinal motility [14].
Barroso [5, 6, 15] demonstrated that there is greater effectiveness of the joint action of essential oils, causing 96, 100 and 100%, in concentrations of 1, 1.5 and 2.0 ml, respectively, in 24 h. A relatively smaller difference when Azadirachta essential oil was used alone indicates that it caused mortality of 65, 97 and 100% at concentrations of 1, 1.5 and 2 ml, respectively. The lowest efficacy observed was that of
Barroso’s studies [5, 6, 15] also corroborate the results of Amer and Mehlhorn [16], where the authors evaluated the larvicidal potential of 41 essential oils, analyzing this effect after 1, 12 and 24 h. More than 48% of these oils only acted after 12 h of exposure, that is, not during the first few hours.
After this approach, it is logical to conclude that the fight against pests will be directed toward sustainability if we fully exploit the properties of
Another fact is the abundance of these vegetables, where we can find them widely distributed, in most continents. This will allow, from an economic point of view, to promote and use more sustainable practices in the management and control of pests in agriculture.
5. Conclusion
After the experiments, the following was concluded:
The essential oils of
The essential oils of
The
No significant effects of the essential oils on the hatching rates of eggs of the three evaluated species were observed, but the bibliography admits this possibility if the concentration of the active principle of azadirachtin or ricin is increased.
References
- 1.
Carson R. Silent Spring. University of Lisbon Press; 1962 - 2.
Carvalho SM, Ferreira DT. Santabárbara against the crowdfunding. Science Today. 1990; 11 (65):65-67 - 3.
Barros R. Botanical Aspects, Traditional Uses, and Potentialities of Azadirachta indica. Pará, Brazil; 2013 - 4.
Martinez SS. Neem, Azadirachtina indica – Nature, Multiple Uses, Production. Londrina: IAPAR; 2002. 142 p - 5.
Barroso AJ. Assessment of the insecticidal potential of Azadirachta indica and Ricinus communis on larvae of Anopheles spp and Aedes spp. Luanda Angola; 2019 - 6.
Barroso AJ. Assessment of the Insecticide Potential of Azadirachta indica and Ricinus communis on Adults of Anopheles spp and Aedes spp. Luanda Angola; 2019 - 7.
Fonseca et al. Toxicity of Ricinus Present in Castor Beans. Belo Horizonte, Brazil: UFMG; 2013 - 8.
Peron F, Ferreira G. Insecticide potential of castor bean seed extract ( Ricinus communis L.) in the control of fall armyworm (Spodoptera frugiperda ). 2012. ISBN 978-85-8084-413-9 - 9.
Santos HO, Mann RS, Powerful JCM, Andrade TM, Alves RA, Ribeiro GT, et al. Efficiency of the aqueous extract of castor bean leaves ( Ricinus communis L .) on eggs and fifth instar nymphs of the predator Podisus nigrispinus dallas (Pentatomidae ). In: V Brazilian Congress of Oil Plants, Oils, Fats and Biodiesel, 2008, Lavras. V Brazilian Congress of Oleaginous Plants, Oils, Fats and Biodiesel. 2008 - 10.
Burg IC, Mayer H. Prevention and Control of Pests and Diseases. Francisco Beltrão: Paraná Grafit; 2000. p. 154 - 11.
Murdue (Luntz) AJ, Blackwell A. Azadirachtin: An update. Journal of Insect Physiology, Oxford. 1993; 39 (11):903-924 - 12.
Góes GB, Neri DKP, Chaves JWN, Maracajá PB. Effect of plant extracts on the control of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Caatinga. 2003; 16 (1/2):47-49 - 13.
Abdel-Shafy S, Zayed AA. In vitro acaricidal effect of plant extract of neem seed oil ( Azadirachta indica ) on egg, immature and adult stages ofHyalomma anatolicum excavatum (Ixodoidea: Ixodidae). Veterinary Parasitology. 2002;106 (1):89-96 - 14.
Viegas J. Terpenes with insecticidal activity: An alternative for the chemical control of insects. New Chemistry. 2003; 26 :390-400 - 15.
Barroso AJ. Evaluation of the Insecticide Potential of Azadirachta indica and Ricinus communis on Diptera Adults and Larvae. Luanda, Angola; 2023 - 16.
Amer A, Mehlhorn H. Larvicidal effects of several essential oils against larvae of Aedes, Anopheles andCulex (Diptera, Culicidae). Parasitology Research. 2006;99 :466-472