Abstract
Heterocyclic ring systems are gaining attention due to their pivotal role in drug design and medicinal chemistry. Quinazolines are nitrogen-containing heterocyclic pharmacophoric units found in abundance in natural and pharmaceutical products. Imidazoquinazolines and benzimidazoquinazolines are fused tricyclic and tetracyclic heterocyclic moieties, respectively. Different isomeric forms of imidazoquinazolines and benzimidazoquinazolines exhibited a plethora of biological applications such as antitumor, antimicrobial, antioxidant, anti-inflammatory, antitubercular, anticancer, antihypertensive, anticonvulsant, antiviral, antimalarial, antiapoptotic, anti-proliferative activities, etc. This chapter addressed the recent synthetic strategies for medicinally privileged scaffolds; imidazoquinazolines and benzimidazoquinazolines. The synthetic routes of various isomeric forms of above-mentioned heterocycles have also been discussed.
Keywords
- Quinazoline
- Imidazoquinazoline
- Benzimidazoquinazoline
- synthetic methodologies
- medicinal importance
1. Introduction
Heterocycles gain much attention because of their vast applications in biology [1, 2, 3] and material sciences [4, 5]. Heterocycles possessed a lot of medicinal benefits [6, 7, 8, 9] and played an important role in drug design and development. Among the heterocycles, quinazoline is a bicyclic
Imidazole and benzimidazole are heterocyclic moieties and important pharmacophores in medicinal chemistry. Imidazole is a five-membered aromatic heterocycle that exhibits a number of biological applications, such as antibacterial [18], anticancer [19], antiepileptic [20], antitubercular [21] activities, etc. Benzimidazoles are privileged structures related to their roles in medicinal chemistry, e.g., they play their role in antibacterial [22], antidiabetic [23], antiviral [24], antiulcer [25] activities, etc. Drugs containing quinazoline, imidazole, and benzimidazole are given in Figure 1.
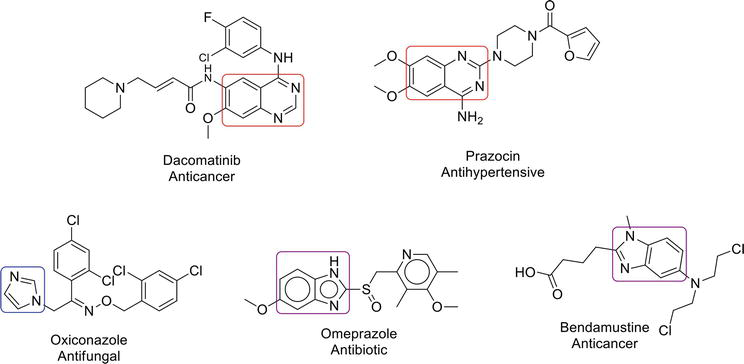
Figure 1.
Quinazoline, imidazole, and benzimidazole-based drugs.
Imidazoquinazoline and benzimidazoquinazoline moieties play significant roles as active biological agents. Imidazoquinazoline I is a PI3K inhibitor [26], imidazoquinazoline II is antiapoptotic [27] and benzimidazoquinazoline III is antitumor [28] in its action (Figure 2).
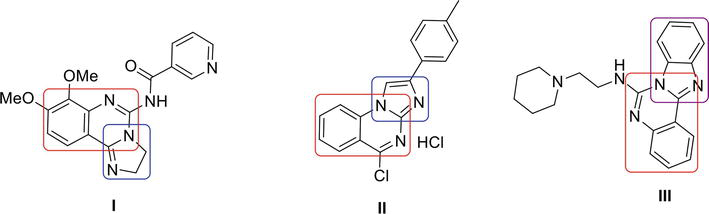
Figure 2.
Biologically active analogues.
2. Imidazoquinazoline
Imidazoquinazoline is a fused tricyclic structure containing imidazole and quinazoline and has three nitrogen atoms in its molecular architecture. It is an important scaffold in drug molecules such as antithrombotic and anticardiotonic agents [29]. Certain derivatives of imidazoquinazolines show a plethora of biological applications, i.e., antitumor [30], anticonvulsant [31], antihypertensive [32], etc.
Structures of imidazoquinazoline and their different isomeric forms are given in Figures 3 and 4.
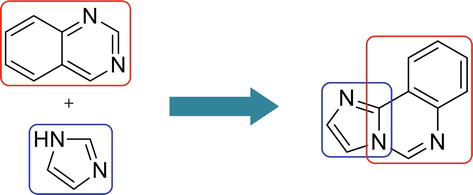
Figure 3.
Structure of Imidazoquinazoline.
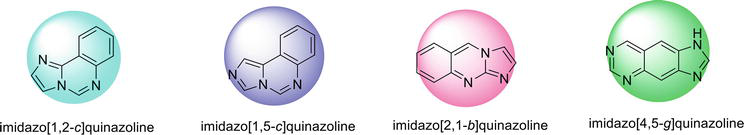
Figure 4.
Different isomeric forms of Imidazoquinazoline.
2.1 Imidazo[1,2-c ]quinazoline
Imidazo[1,2-
2.1.1 One-pot exhaustive dehydrogenation
An expedient way to synthesize imidazo [1,2-
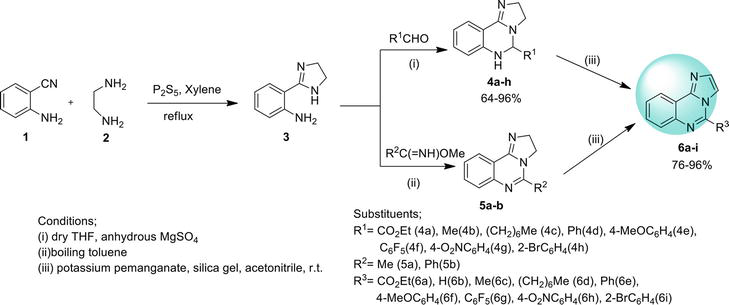
2.1.2 Ullmann coupling reaction
Imidazo[1,2-
2.1.3 Conventional or oxidative coupling
Imidazo[1,2-
2.1.4 Intramolecular C-N coupling
Imidazo[1,2-
2.2 Imidazo[1,5-c ]quinazline
Imidazo[1,5-
2.2.1 Oxidative domino sp3 C–H amination
An oxidative domino synthesis pathway for the formation of imidazo [1,5-
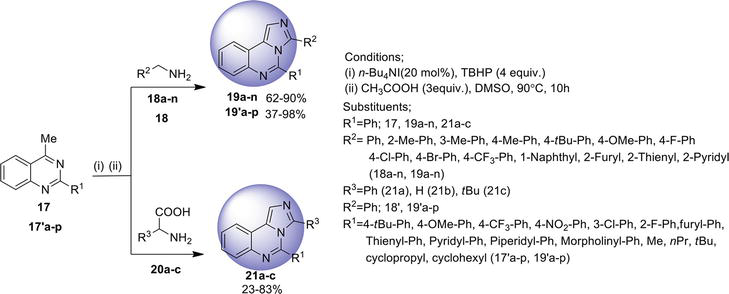
2.3 Imidazo[2,1-b ]quinazoline
Imidazo[2,1-
2.3.1 Cascade reaction
A cascade microwave-promoted reaction involving Claisen-Schmidt, aza-Michael, and cyclization reactions in the formation of imidazo[2,1-
2.4 Imidazo[4,5-g ]quinazoline
Imidazo[4,5-
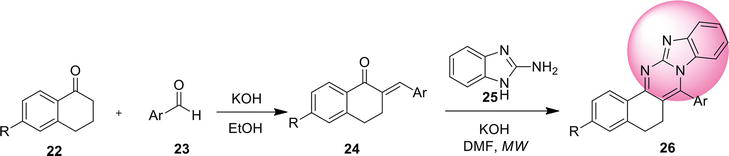
2.4.1 Reductive coupling
Imidazo[4,5-
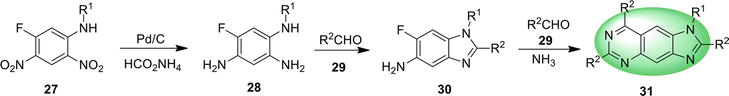
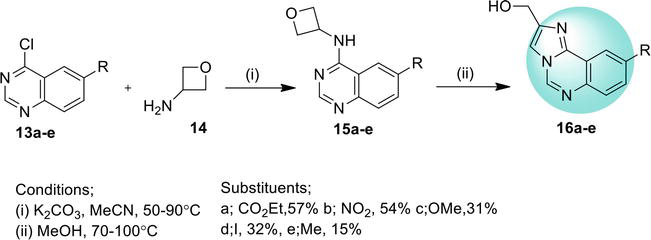
3. Benzimidazoquinazoline
Benzimidazoquinazoline is a fused tetracyclic structure containing benzimidazole and quinazoline, having three nitrogen atoms in its molecular architecture. Benzimidazoquinazoline derivatives act as potent immunosuppressors [54] and possess promising antitumor activity [55] as well.
Structures of benzimidazoquinazoline and their different isomeric forms are given in Figures 5 and 6.
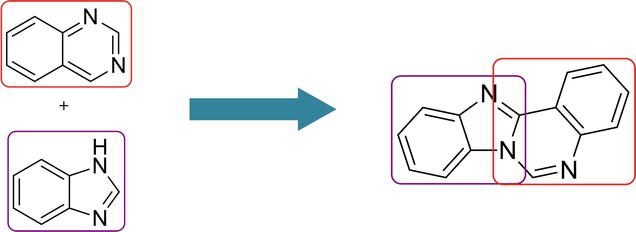
Figure 5.
Structure of benzimidazoquinazoline.
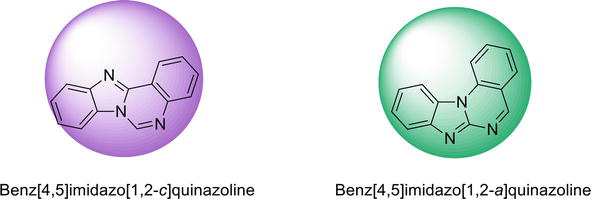
Figure 6.
Different isomeric forms of benzimidazoquinazoline.
3.1 Benz[4,5]imidazo[1,2-c ]quinazoline
Benzo[4,5]imidazo[1,2-
3.1.1 Conventional or oxidative coupling
Benz[4,5]imidazo[1,2-
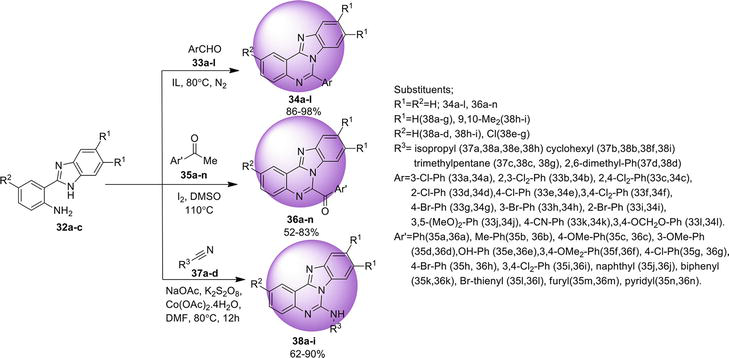
A domino synthetic approach for benz[4,5]imidazo[1,2-
3.1.2 Intramolecular C-N coupling via C-X activation
Another approach to synthesize benz[4,5]imidazo[1,2-
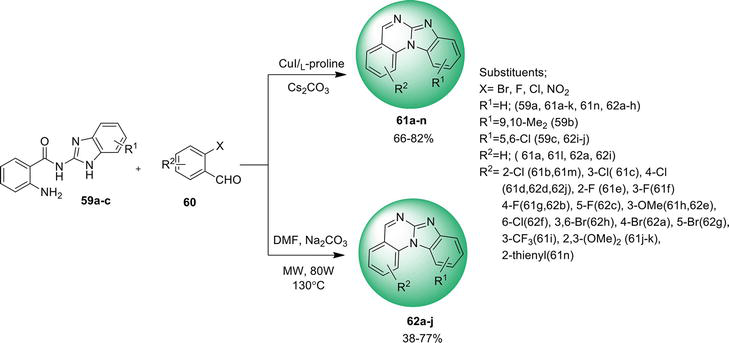
3.1.3 Ullmann N -arylation reaction
An easy way found to synthesize benz[4,5]imidazo[1,2-
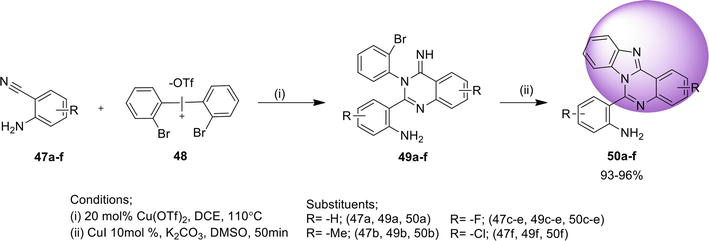
3.1.4 Directed arylic C-H amidation
Another efficient protocol reported to synthesize benz[4,5]imidazo[1,2-
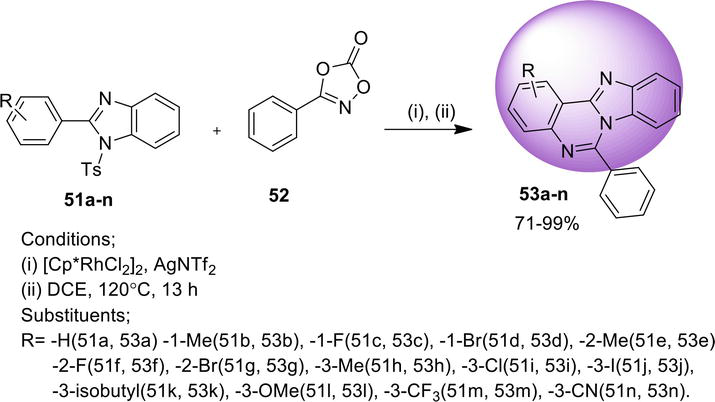
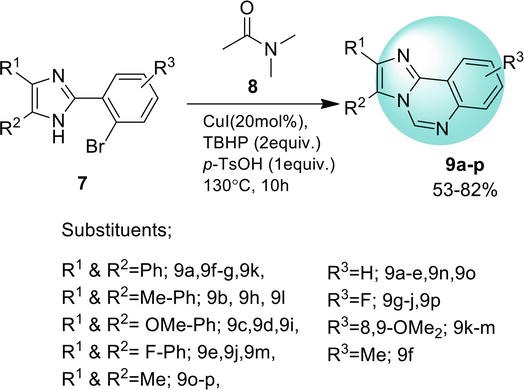
3.1.5 Double C-H functionalization
A metal-free pathway for the synthesis of benz[4,5]imidazo[1,2-
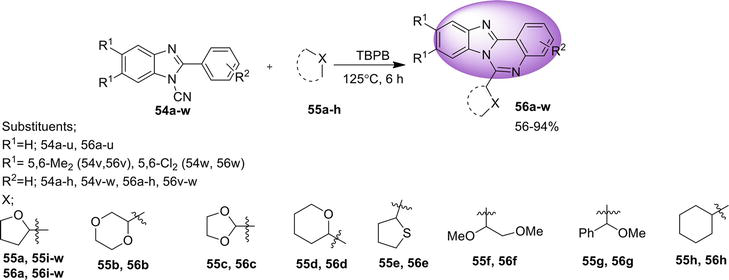
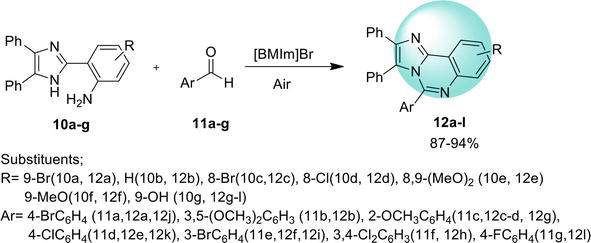
3.2 Benz[4,5]imidazo[1,2-a ]quinazoline
Here are the methods for the synthesis of another isomer, benz[4,5]imidazo[1,2-
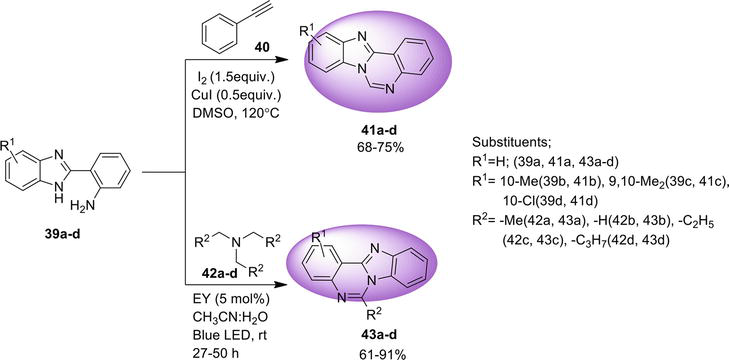
3.2.1 Transition metal-free tandem process
One-pot regioselective synthesis of benz[4,5]imidazo[1,2-
3.2.2 Intramolecular C: N bond formation
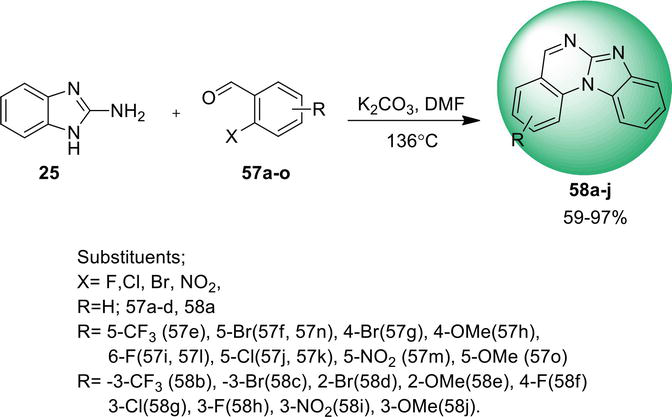
Benz[4,5]imidazo[1,2-
4. Conclusion
The fused heterocyclic moieties tend to achieve the top of the list position in drug design because of their wide range of pharmacological applications. In this context, this chapter is focused on the different synthetic approaches of imidazoquinazolines and benzimidazolquinazolines. These biological scaffolds were prepared by a large number of synthetic routes that are summarized in this chapter. These routes include Ullmann cross-coupling reaction, Claisen–Schmidt reaction, Aza–Michael reaction, cyclization reaction, Cu(OTf)2 catalyzed reaction, Iodine-mediated oxidative annulation reaction, oxidative and nonoxidative C-N coupling reaction, photoredox catalyzed synthesis, metal-free synthesis, green synthesis, microwave mediated synthesis, oxidative domino synthesis, transition metal-free and transition metal-catalyzed tandem processes. These synthetic strategies will be helpful in bringing novelty to the synthesis of bioactive compounds and in exploring a new area of medicinal research.
References
- 1.
Aslam S, Zaib S, Ahmad M, Gardiner JM, Ahmad A, Hameed A, et al. Novel structural hybrids of pyrazolobenzothiazines with benzimidazoles as cholinesterase inhibitors. European Journal of Medicinal Chemistry. 2014; 78 :106-117 - 2.
Ahmad M, Siddiqui LH, Gardiner JM, Parvez M, Aslam S. Synthesis and antioxidant studies of novel N -substituted-benzyl/phenyl-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c ][1,2]benzothiazin-2(4H )-yl)acetamides. Medicinal Chemistry Research. 2012;22 :794-805 - 3.
Aslam S, Ahmad M, Zia-ur-rehman M, Montero C, Detorio M, Parvez M, et al. Synthesis and anti-HIV-1 screening of novel N′ -(1-(aryl)ethylidene)-2-(5,5-dioxido-3-phenylbenzo[e ]pyrazolo[4,3-c ][1,2]thiazin-4(1H )-yl)acetohydrazides. Archives of Pharmacal Research. 2013;37 :1380-1393 - 4.
Li J, Li P, Wu J, Gao J, Xiong W, Zhang G, et al. [4 + 2] cycloaddition reaction to approach Diazatwistpentacenes: Synthesis, structures, physical properties, and self-assembly. The Journal of Organic Chemistry. 2014; 79 :4438-4445 - 5.
Gu P, Zhang J, Long G, Zhang Q. Solution-processable thiadiazoloquinoxaline-based donor-acceptor small molecules for thin-film transistors. Journal of Materials Chemistry C. 2016; 4 :3809-3814 - 6.
Küçükgüzel SG, Süzgün PC. Recent advances bioactive 1,2,4-triazole-3-thiones. European Journal of Medicinal Chemistry. 2015; 97 :830-870 - 7.
Rizvi SUF, Siddiqui HL, Parvez M, Ahmad M, Siddiqui WA, Yasinzai MM. Antimicrobial and Antileishmanial studies of novel (2 E ) -3- (2-Chloro-6- methyl/methoxyquinolin-3-yl) -1- (aryl) prop-2-en-1-ones. Chemical and Pharmaceutical Bulletin. 2010;58 :301-306 - 8.
Kanwal A, Saddique FA, Aslam S, Ahmad M, Zahoor AF, Mohsin N. Benzimidazole ring system as a privileged template for anticancer agents. Pharmaceutical Chemistry Journal. 2018; 51 :1068-1077 - 9.
Ahmad M, Aslam S, Bukhari MH, Montero C, Detorio M, Parvez M, et al. Synthesis of novel pyrazolobenzothiazine 5 , 5-dioxide derivatives as potent anti-HIV-1 agents. Medicinal Chemistry Research. 2013; 23 :1309-1319 - 10.
Gineinah MM, El-Sherbeny MA, Nasr MN, Maarouf AR. Synthesis and antiinflammatory screening of some quinazoline and quinazolyl-4-oxoquinazoline derivatives. Archiv der Pharmazie: An International Journal Pharmaceutical and Medicinal Chemistry. 2003; 335 :556-562 - 11.
Jafari E, Khajouei MR, Hassanzadeh F, Hakielahi GH, Khodarahmi GA. Quinazolinone and quinazoline derivatives: Recent structures with potent antimicrobial and cytotoxic activities. Research in Pharmaceutical Sciences. 2016; 11 :1-14 - 12.
Ugale VG, Bari SB. Quinazolines: New horizons in anticonvulsant therapy. European Journal of Medicinal Chemistry. 2014; 80 :447-501 - 13.
Rojas-Aguirre Y, Hernández-Luis F, Mendoza-Martínez C, Sotomayor CP, Aguilar LF, Villena F, et al. Effects of an antimalarial quinazoline derivative on human erythrocytes and on cell membrane molecular models. Biochimica et Biophysica Acta - (BBA) Biomembranes. 2012; 1818 :738-746 - 14.
Kuneš J, Bazant J, Pour M, Waisser K, Šlosárek M, Janota J. Quinazoline derivatives with antitubercular activity. Il Farmaco. 2000; 55 :725-729 - 15.
Manasa K, Sidhaye RV, Nalini CN. Synthesis, antioxidant and anticancer activity of quinazoline derivatives. Journal of Current Pharma Research. 2011; 1 :7842 - 16.
Rahman MU, Rathore A, Siddiqui AA, Parveen G, Yar MS. Synthesis and characterization of quinazoline derivatives : Search for hybrid molecule as diuretic and antihypertensive agents. Journal of Enzyme Inhibition and Medicinal Chemistry. 2014; 29 :733-743 - 17.
Bianco A, Reghellin V, Donnici L, Fenu S, Alvarez R, Baruffa C, et al. Metabolism of phosphatidylinositol 4-kinase IIIα-dependent PI4P is subverted by HCV and is targeted by a 4-Anilino Quinazoline with antiviral activity. PLoS Pathogens. 2012; 8 :e1002576 - 18.
Rossi R, Ciofalo M. An updated review on the synthesis and antibacterial activity of molecular hybrids and conjugates bearing imidazole moiety. Molecules. 2020; 25 :5133 - 19.
Sharma P, Larosa C, Antwi J, Govindarajan R, Werbovetz KA. Imidazoles as potential anticancer agents: An update on recent advances. Molecules. 2021; 26 :4213 - 20.
Mishra R, Ganguly S. Imidazole as an anti-eoileptic: An overview. Medicinal Chemistry Research. 2012; 21 :3929-3939 - 21.
Fan YL, Jin XH, Huang ZP, Yu HF, Zeng ZG, Gao T, et al. Recent advances of imidazole-containing derivatives as anti-tubercular agents. European Journal of Medicinal Chemistry. 2018; 150 :347-365 - 22.
Song D, Ma S. Recent development of Benzimidazole-containing antibacterial agents. ChemMedChem. 2016; 11 :646-659 - 23.
Ibraheem F, Ahmad M, Ashfaq UA, Aslam S, Khan ZA, Sultan S. Synthesis, molecular docking and anti-diabetic studies of novel benzimidazole-pyrazoline hybrid molecules. Pakistan Journal of Pharmaceutical Sciences. 2020; 33 :847-854 - 24.
Kanwal A, Ahmad M, Aslam S, Naqvi SAR, Saif MJ. Recent advances in antiviral benzimidazole derivatives: a mini review. Pharmaceutical Chemistry Journal. 2019; 53 :179-187 - 25.
Patil A, Ganguly S, Surana S. A systematic review of benzimidazole derivatives as an antiulcer agent. Rasayan Journal of Chemistry. 2008; 1 :447-460 - 26.
Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, et al. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell. 2006; 125 :733-747 - 27.
End DW, Mevellec L, Angibaud P. Farnesyl protein transferase inhibitors: Medicinal chemistry, molecular mechanisms, and progress in the clinic. Cancer. 2006; 1 :133-168 - 28.
Perron D, Conlon D, Bousquet PF, Robinson SP. Benzimidazo[1,2- c ]quinazoline dimers as potential antitumor agents. Journal of Heterocyclic Chemistry. 1997;34 :807-812 - 29.
Silverstein MN, Petitt RM, Solberg LA Jr, Fleming JS, Knight RC, Schacter LP. Anagrelide: A new drug for treating thrombocytosis. New England Journal of Medicine. 1988; 318 :1292-1294 - 30.
Ye W, Liu Y, Ren Q, Liao T, Chen Y, Chen D, et al. Design, synthesis and biological evaluation of novel triazoloquinazolinone and imidazoquinazolinone derivatives as allosteric inhibitors of SHP2 phosphatase. Journal of Enzyme Inhibition and Medicinal Chemistry. 2022; 37 :1495-1513 - 31.
Jackson HC, Hansen HC, Kristiansen M, Suzdak PD, Klitgaard H, Judge ME, et al. Anticonvulsant profile of the imidazoquinazolines NNC 14-0185 and NNC 14-0189 in rats and mice. European Journal of Pharmacology. 1996; 308 :21-30 - 32.
Chern J-W, Tao P-L, Yen M-H, Guan-Yu L, Shiau C-Y, Lai Y-J, et al. 2,3-Dihydroimidazo[l,2- c ]quinazoline derivatives: A novel class of potent and selective al-Adrenoceptor antagonists and anti hypertensive agent. Journal of Medicinal Chemistry. 1993;36 :2196-2207 - 33.
Balakumar C, Lamba P, Kishore DP, Narayana BL, Rao KV, Rajwinder K, et al. Synthesis, anti-inflammatory evaluation and docking studies of some new fluorinated fused quinazolines. European Journal of Medicinal Chemistry. 2010; 45 :4904-4913 - 34.
Aly AA. Synthesis and antimicrobial activity of some annelated quinazoline derivatives. Journal of the Chinese Chemical Society. 2007; 54 :437-446 - 35.
Barchéchath SD, Tawatao RI, Corr M, Carson DA, Cottam HB. Inhibitors of apoptosis in lymphocytes: Synthesis and biological evaluation of compounds related to pifithrin-α. Journal of Medicinal Chemistry. 2005; 48 :6409-6422 - 36.
Helali AY, Sarg MT, Koraa MM, El-Zoghbi MS. Utility of 2-methyl-quinazolin-4 (3 H )-one in the synthesis of heterocyclic compounds with anticancer activity. Open Journal of Medicinal Chemistry. 2014;4 :44227 - 37.
Stoessel P, Heil H, Jooseten D, Pfumm C, Gerhard A, Breuning E. Cyclometalated transition metal complexes with condensed Polyheterocyclic bidentate ligands as dopants for organic electroluminescent devices. Chemical Abstracts. 2010; 2010 (153):27153 - 38.
Korshin EE, Sabirova LA, Levin YA. An expedient synthesis of 5-substituted imidazo [1, 2- c ] quinazolines. Synthesis. 2012;44 :3512-3522 - 39.
Claudi F, Franchetti P, Grifantini M, Martelli S. Isomerization of 4-(1-aziridinyl) quinazolines to 2, 3-dihydroimidazo [1, 2- c ] quinazolines. The Journal of Organic Chemistry. 1974;39 :3508-3511 - 40.
Nandwana NK, Dhiman S, Saini HK, Kumar I, Kumar A. Synthesis of Quinazolinones, Imidazo [1,2- c ] quinazolines and Imidazo[4,5-c ]quinolines through tandem reductive amination of aryl halides and oxidative amination of C (sp3)–H bonds. European Journal of Organic Chemistry. 2017;2017 :514-522 - 41.
Nandwana NK, Shinde VN, Saini HK, Kumar A. Copper-catalyzed one-pot tandem reaction for the synthesis of Imidazo [1, 2- c ][1, 2, 3] triazolo [1, 5-a ] quinazolines. European Journal of Organic Chemistry. 2017;2017 :6445-6449 - 42.
Nandwana NK, Singh RP, Patel OP, Dhiman S, Saini HK, Jha PN, et al. Design and synthesis of imidazo/benzimidazo [1, 2- c ] quinazoline derivatives and evaluation of their antimicrobial activity. ACS Omega. 2018;3 :16338-16346 - 43.
Qiang WW, Zhang MM, Wang XS. Catalyst-free synthesis of 5-Arylimidazo [1,2- c ] quinazoline derivatives in ionic liquids. Journal of Heterocyclic Chemistry. 2017;54 :509-516 - 44.
Wu H, Wang YC, Li TJ, Liu JQ, Wang XS. A Cascade synthesis of 11 bH-Imidazo [1, 2- c ] isoquinolino [2, 1-a ] quinazoline derivatives catalyzed by AgOTf. Journal of Heterocyclic Chemistry. 2020;57 :2203-2212 - 45.
Bagal S, Bodnarchuck M, King T, Mckerrecher D, Luo X, Wang P, et al. SN Ar reaction of Aminooxetanes with Quinazolines: Synthesis of Imidazo[1,2- c ]quinazolines. Synlett. 2020;31 :502-506 - 46.
Khoza TA, Makhafola TJ, Mphahlele MJ. Novel polycarbo-substituted imidazo [1, 2- c ] quinazolines: Synthesis and cytotoxicity study. Molecules. 2015;20 :22520-22533 - 47.
Zhao D, Wang T, Shen Q, Li JX. n -Bu4NI-catalyzed selective dual amination of sp3 C–H bonds: Oxidative domino synthesis of imidazo [1, 5-c ] quinazolines on a gram-scale. Chemical Communications. 2014;50 :4302-4304 - 48.
Itoh T. Fluorescence and phosphorescence from higher excited states of organic molecules. Chemical Reviews. 2012; 112 :4541-4568 - 49.
Hirano K, Oderaotoshi Y, Minakata S, Komatsu M. Unique fluorescent properties of 1-aryl-3, 4-diphenylpyrido [1, 2- a ] benzimidazoles. Chemistry Letters. 2001;30 :1262-1263 - 50.
Devi Priya D, Mohana RS. Claisen-Schmidt, aza-Michael, cyclization via cascade strategy toward microwave promoted synthesis of imidazo [2,1- b ] quinazolines. Synthetic Communications. 2020;50 :1813-1834 - 51.
Devipriya D, Roopan SM. UV-light intervened synthesis of imidazo fused quinazoline and its solvatochromism, antioxidant, antifungal and luminescence properties. Journal of Photochemistry and Photobiology B: Biology. 2019; 190 :42-49 - 52.
Li L, Zhang Q, Liu B, Liu G. A novel multi-component reaction to imidazo [4,5- g ]-quinazolines. Molecules. 2013;18 :5697-5705 - 53.
Rewcastle GW, Palmer BD, Bridges AJ, Showalter HH, Sun L, Nelson J, et al. Tyrosine kinase inhibitors. 9. Synthesis and evaluation of fused tricyclic quinazoline analogues as ATP site inhibitors of the tyrosine kinase activity of the epidermal growth factor receptor. Journal of Medicinal Chemistry. 1996; 39 :918-928 - 54.
Lunn WH, Harper RW, Stone RL. Benzimidazo [2,1- b ] quinazolin-12-ones. New class of potent immunosuppressive compounds. Journal of Medicinal Chemistry. 1971;14 :1069-1071 - 55.
de Tako Tsubo OF. View metadata, citation and similar papers at core. Archives of Cardiovascular Diseases Supplements. 2013; 5 :1-18 - 56.
Rohini R, Shanker K, Reddy PM, Ravinder V. Synthesis and antimicrobial activities of a new class of 6-arylbenzimidazo [1,2- c ] quinazolines. Journal of the Brazilian Chemical Society. 2010;21 :49-57 - 57.
Dwyer MP, Keertikar KM, Chen L, Tong L, Selyutin O, Nair AG, et al. Matched and mixed cap derivatives in the tetracyclic indole class of HCV NS5A inhibitors. Bioorganic & Medicinal Chemistry Letters. 2016; 26 :4106-4111 - 58.
Lamazzi C, Léonce S, Pfeiffer B, Renard P, Guillaumet G, Rees CW, et al. Expeditious synthesis and cytotoxic activity of new cyanoindolo [3,2- c ] quinolines and benzimidazo [1,2-c ] quinazolines. Bioorganic & Medicinal Chemistry Letters. 2000;10 :2183-2185 - 59.
Wolfe JF, Rathman TL, Sleevi MC, Campbell JA, Greenwood TD. Synthesis and anticonvulsant activity of some new 2-substituted 3-aryl-4 (3 H )-quinazolinones. Journal of Medicinal Chemistry. 1990;33 :161-166 - 60.
Galarce GD, Foncea RE, Edwards AM, Pessoa-Mahana H, Pessoa-Mahana CD, Ebensperger RA. Biological evaluation of novel 6-Arylbenzimidazo [1,2- c ] quinazoline derivatives as inhibitors of LPS-induced TNF-alpha secretion. Biological Research. 2008;41 :43-50 - 61.
Chen DS, Liu SJ, Lu WQ, Wang XS. Green synthesis of 6-Aryl-5, 6-dihydrobenzo [4, 5] imidazo [1, 2- c ] quinazoline derivatives in ionic liquid under catalyst-free conditions. Journal of Heterocyclic Chemistry. 2018;55 :166-172 - 62.
Ambethkar S, Kalaiselvi M, Ramamoorthy J, Padmini V. I2-catalyzed oxidative cross-coupling reaction of methyl ketones and 2-(2-aminophenyl) benzimidazole: Facile access to benzimidazo [1,2- c ] quinazoline. ACS Omega. 2018;3 :5021-5028 - 63.
Ahmadi F, Bazgir A. Synthesis of benzoimidazoquinazolines by cobalt-catalyzed isocyanide insertion–cyclization. RSC Advances. 2016; 6 :61955-61958 - 64.
Donthiboina K, Mani GS, Shankaraiah N, Kamal A. Iodine-mediated oxidative annulation by C–C cleavage: A domino synthetic approach to Quinazolinones and benzo [4,5] imidazo [1, 2- c ] quinazolines. Chemistry Select. 2020;5 :3923-3928 - 65.
Arumugam A, Palani P, Anandan M, Nutalapati V, Senadi GC. Metal-free, Photoredox-catalyzed synthesis of Quinazolin-4 (3 H )-ones and benzo [4,5] imidazo [1, 2-c ] quinazolines using Trialkylamines as alkyl synthon. European Journal of Organic Chemistry. 2023;26 :e202300100 - 66.
Mirallai SI, Koutentis PA. The Conversion of 4-Anilinoquinazoline-and 3-Aryl-4-imino-3, 4-dihydro-quinazoline-2-carbonitriles into Benzo [4, 5] imidazo [1,2- c ] quinazoline-6-carbonitriles via Oxidative and Nonoxidative C–N Couplings. The Journal of Organic Chemistry. 2015;80 :8329-8340 - 67.
Shen C, Wang L, Wen M, Shen H, Jin J, Zhang P. Synthesis of benzimidazo [1, 2- c ] quinazolines via metal-free intramolecular C–H amination reaction. Industrial & Engineering Chemistry Research. 2016;55 :3177-3181 - 68.
Pang X, Chen C, Li M, Xi C. A concise and efficient synthesis of benzimidazo [1, 2- c ] quinazolines through CuI-catalyzed intramolecular N-arylations. Beilstein Journal of Organic Chemistry. 2015;11 :2365-2369 - 69.
Xu L, Li T, Wang L, Cui X. Rh (III)-catalyzed one-pot synthesis of Benzimidazoquinazolines via C–H Amidation–cyclization of N -LG-2-phenylbenzoimidazoles. The Journal of Organic Chemistry. 2018;84 :560-567 - 70.
Wu X, Sun S, Xu S, Cheng J. Rh-catalyzed annulation of ortho-C−H bonds of 2-Arylimidazoles with 1, 4, 2-Dioxazol-5-ones toward 5-Arylimidazo [1, 2- c ] quinazolines. Advanced Synthesis & Catalysis. 2018;360 :1111-1115 - 71.
Tian X, Fan Z, Jiang S, Li Z, Li J, Zhang Y, et al. Metal-free synthesis of Benzimidazo [1, 2- c ] quinazolines fromN -Cyanobenzimidazoles via double C—H Functionalizations. Chinese Journal of Organic Chemistry. 2022;42 :3684 - 72.
Jiang S, Tian XJ, Feng SY, Li JS, Li ZW, Lu CH, et al. Visible-light photoredox catalyzed double C–H functionalization: Radical cascade cyclization of ethers with benzimidazole-based cyanamides. Organic Letters. 2021; 23 :692-696 - 73.
Fang S, Niu X, Yang B, Li Y, Si X, Feng L, et al. One-pot synthesis of benzo [4, 5] imidazo [1,2- a ] quinazoline derivatives via facile transition-metal-free tandem process. ACS Combinatorial Science. 2014;16 :328-332 - 74.
Kim MJ, Lee SW, Dao PD, Cho CS. Synthesis of benzo [4,5] imidazo [1, 2- a ] indolo [1, 2-c ] quinazolines from 2-(2-bromoaryl) indoles and 2-methoxybenzimidazoles under recyclable magnetic MOF-199 catalysis. Applied Organometallic Chemistry. 2022;36 :e6871 - 75.
Li C, Zhang WT, Wang XS. CuI-catalyzed C–N bond formation and cleavage for the synthesis of Benzimidazo [1, 2- a ] quinazoline derivatives. The Journal of Organic Chemistry. 2014;79 :5847-5851 - 76.
Kumar P, Singh AK, Bahadur V, Len C, Richards NG, Parmar VS, et al. Microwave-assisted, metal-free, base-mediated C–N bond formation/cleavage: Synthesis of Benzimidazo [1, 2- a ] quinazoline derivatives. ACS Sustainable Chemistry & Engineering. 2016;4 :2206-2210