Abstract
Pulmonary arterial hypertension is a disease characterized by complex pathogenesis and high mortality rates following diagnosis. Non-coding RNA plays a pivotal role in the development of pulmonary arterial hypertension, offering promising prospects as a diagnostic and therapeutic target for this condition. The utilization of nucleic acid drugs in disease treatment suggests the feasibility of packaging non-coding RNA into carrier systems and employing them in human pulmonary arterial hypertension (PAH) treatment through appropriate delivery routes. However, currently, no nucleic acid drugs are available for the clinical treatment of PAH. Identifying active regions within non-coding RNA through molecular docking analysis and developing suitable nucleic acid drugs hold great potential for advancing the field of PAH therapeutics.
Keywords
- non-coding RNA
- delivery
- molecular docking
- pulmonary arterial hypertension
- clinical application
1. Introduction
1.1 Non-coding RNAs and pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is a grave ailment characterized by the elevation in pulmonary artery pressure (mean pulmonary artery pressure ≥ 25 mmHg at rest) [1, 2], which results from the changes in the structure and function of the vessel wall due to aberrant proliferation, apoptosis, and migration of cells [3]. The causes of PAH can be broadly classified into primary and secondary causes. Currently, the pathogenesis of PAH is known to involve ion channels, vasoactive substances, immune factors, and genetic factors [4, 5, 6].
Recently, a growing body of evidence has highlighted the crucial regulatory roles played by non-coding RNAs (ncRNAs) in PAH development. It is worth noting that the majority of human genes (>95%) produce ncRNAs. MicroRNAs (miRNAs), which consist of 21–22 nucleotides, exert post-transcriptional control over gene expression [7]. Long non-coding RNAs (lncRNAs) are transcribed from intergenic or intragenic regions and typically exceed 200 nucleotides in length (Figure 1). They possess the ability to interact with proteins, RNA molecules, or DNA sequences to fulfill their regulatory functions [9]. Circular RNAs (circRNAs) represent a novel class of ncRNAs characterized by their closed-loop structure, which confers exceptional stability and enables interactions with both proteins and RNA molecules [10].

Figure 1.
RNA classification [
NcRNAs have been identified as regulators of multiple stages in gene expression. However, due to their vast quantity and diverse mechanisms, comprehensively understanding the roles of ncRNAs is challenging. NcRNAs often exhibit cell specificity in expression level and function, which are used for various disease therapy including PAH. A successful ncRNA-based strategy is based on a comprehensive understanding of the regulatory relationships involving ncRNAs [11].
1.2 Patential applications of non-coding RNAs
1.2.1 As therapeutic targets
With ever-increasing advancements in RNA structure determination methods and the ever-expanding discoveries of novel RNA structures and cellular functions, RNA-based therapeutics are emerging as promising approaches for treating human diseases. In general, RNA-based therapeutics are categorized into two types: (1) therapeutic RNAs: RNAs target RNA, DNA, or protein to modulate their function, such as RNA aptamers, antisense oligonucleotides (ASO), small interfering RNAs (siRNA), and guide RNAs (gRNA). Therapeutic RNAs are currently undergoing active development in the field of gene therapy [12, 13, 14, 15]. (2) Targeted RNAs: RNAs serve as a target for binding with drug-like small molecules [15, 16], which bears resemblance to drug discovery targeting proteins.
However, only approximately 1.5% of the human genome is responsible for protein coding [17, 18, 19, 20, 21]. Among these protein-encoding genes, only a small fraction of about 10–15% are associated with diseases [18, 19, 20, 22, 23, 24]. Therefore, RNAs exhibit a much broader druggability than proteins. NcRNAs play crucial roles in various human diseases. Targeting ncRNAs will provide huge opportunities for drug discovery.
Gene therapy is a complex approach to treating diseases, which was first proposed by Theodore Friedmann and Richard Roblin in 1972 in “Science”. RNAs, as a new class of treatments, may be an alternative to DNA therapy in preventing diseases. RNAs function in the cellular cytosol, avoiding the potential risks of insertion into patient genomes. RNAs have demonstrated therapeutic potential primarily through siRNA and microRNA (miRNA) applications, as evidenced by previous research efforts [25, 26]. In recent developments, mRNA vaccines have emerged as a powerful tool in combating infectious diseases such as COVID-19 [27, 28, 29].
NcRNAs have the potential to be PAH therapeutic targets. MiR-140-5p was found to be downregulated in PAH model rats; miR-140-5p mimics prevented the progression of established PAH in rats and suppressed the proliferation and migration of pulmonary artery smooth muscle cells (PASMCs) [30]. The results are promising. However, the clinical application of ncRNA therapy is currently constrained by several factors, including the diverse biological functions of ncRNAs and challenges in formulation and delivery approaches. As a therapeutic agent, ncRNAs may elicit adverse effects that could pose life-threatening risks or result in inadequate deposition within the deep lung tissues. Furthermore, large-scale, comprehensive, and well-designed clinical studies are indispensable for shedding more light on the potential applications of ncRNAs.
1.2.2 As biomarkers
NcRNAs have emerged as potential non-invasive and more sensitive diagnostic biomarkers for PAH. In the early diagnosis of PAH, hsa-miR-21-3p and hsa-miR-143-3p in peripheral plasma can be considered as potential biomarkers [31].
1.3 Status of pulmonary non-coding RNAs delivery
RNA therapeutics have been hailed as the medicine of the future, and this vision has now become our reality [32]. Currently, there are two mRNA vaccines available in the market, along with four siRNA-based therapeutics and five ASO-based drugs [33]. This signifies a true revolution in the field of RNA therapeutics; however, one major obstacle remains delivery [34]. Nanocarriers that are optimized for protecting and delivering payloads of RNA tend to accumulate primarily in the liver when administered intravenously [35]. To overcome this limitation and explore alternative administration routes beyond liver targeting, numerous clinical trials have investigated different approaches for RNA delivery [36]. Intravitreal injection (fomivirsen) and intrathecal injection (nusinersen) have demonstrated success, prompting further exploration of local administration routes such as intranasal or pulmonary delivery through ongoing clinical trials. However, no approved products utilizing these routes have emerged thus far [37]. The lung presents an array of currently untargetable sites that hold potential for treatment using ncRNA therapeutics.
In this chapter, we conducted a comprehensive literature review to identify functional ncRNAs, particularly miRNAs, circRNAs, and lncRNAs associated with PAH. Additionally, we identified potential untapped biomarkers and therapeutic targets for ncRNAs in PAH.
2. MicroRNAs
2.1 Introduction
MicroRNAs (miRNAs) are a class of ncRNAs, approximately 20–24 nucleotides in length, that possess regulatory functions. MiRNA genes are transcribed by RNA polymerase II or III into primary miRNA transcripts (pri-miRNA). These pri-miRNAs are subsequently cleaved by the Drosha-DGCR8 (DiGeorge syndrome critical region 8) complex [38], an RNase III enzyme. The resulting precursor miRNAs (pre-miRNAs) are then transported to the cytoplasm and processed into double-stranded mature miRNAs by the RNase Dicer [39]. This double-stranded form is rapidly loaded onto the RNA-induced silencing complex (RISC), where one of the single strands undergoes degradation while the other mature single-stranded miRNA molecule either degrades or translates its target gene through complementary pairing with the 3′UTR region of said target gene [40].
The stability of miRNAs is remarkably high in both DNA and RNA lysates, making them resistant to degradation. MiRNAs exhibit conserved sequences and functions across diverse cells, tissues, organs, and genera. The distinct expression profiles of miRNAs in various tissues and cells render them valuable molecular markers for specific cellular populations. Importantly, the presence of a particular miRNA at a specific stage within a given cell determines its differentiation direction and phase. Consequently, miRNAs play crucial roles as pivotal regulators governing cell timing and directional differentiation.
Numerous studies have demonstrated that miRNAs exert their biological function by participating in the regulation of downstream gene translation processes, with target mRNAs located within its 3′UTR [41]. In animals, a single miRNA can recognize multiple mRNA targets, and conversely, one mRNA target can be recognized by multiple miRNAs. It is estimated that approximately two-thirds of protein-coding genes in the human genome are regulated by miRNAs.
2.2 MiRNAs and PAH
It is widely acknowledged that miRNA dysfunction contributes to the enhanced proliferation of endothelial cells, smooth muscle cells, and outer epidermal cells in PAH. Increasing evidence suggests that various miRNAs and their expression changes (either upregulation or downregulation) are associated with PAH pathogenesis. MiRNAs regulate the proliferation and apoptosis of pulmonary artery endothelial cells (PAECs), PASMCs, and extrapulmonary fibroblasts through their target genes. They play roles in all aspects of pulmonary vasoconstriction and reconstruction. However, due to a large number of publications based on diverse experimental methods, it remains challenging to identify the most crucial miRNAs as potential therapeutic targets for PAH [42].
The level of miR-130/301 has been shown to be elevated in both pulmonary vessels and plasma in mammalian models as well as human patients with PAH. MiR-130/301 promotes PAH-related phenotypes through the apelin-miR-424/503-FGF2 signaling pathway in endothelial cells, while it utilizes the STAT3/miR-204 signaling pathway in smooth muscle cells. Administration of miR-130a oligonucleotide mimics enhances pathogenic effects related to PAH, whereas inhibition of miR-130/301 prevents the onset of PAH in mice treated with SU5416 under hypoxic conditions [43].
The miRNA-17/92 cluster exerts an impact on the proliferation of PAECs and PASMCs. The combination of miR-17/18a/19a/92a demonstrates the most potent induction effect on PCNA and α-SMA, while maintaining calponin and SM22α levels. Consequently, in SM-17 ~ 92−/− mice, miR-17 to 92 was reconstituted by administering a mixture of miR-17/18A/19a/92a mimics via intravenous injection through the tail vein. MiR-17 to 92 reconstitution restored elevated right ventricular systolic blood pressure (RVSP), RV/(LV + S) ratios, and pulmonary artery remodeling in hypoxia-exposed SM-17 ~ 92−/− mice without altering basal levels of RVSP, RV/(LV + S), or pulmonary artery wall thickness. These findings suggest that miR-17 ~ 92 plays a crucial role in the pathogenesis of PAH. By investigating PASMCs and lung tissue from PAH patients, it has been demonstrated that miRNA-17/92 promotes PASMC proliferation through the PDLIM5/TGF-beta/Smad pathway, offering novel insights for PAH treatment [44].
The expression of miR-495 is significantly upregulated in the mouse model of PAH induced by chronic hypoxia and hypoxia-Su5416 (HySu). Inhibition of miR-495 via adeno-associated virus type 9 (AAV9) effectively mitigates hemodynamic abnormalities and structural changes in the pulmonary vasculature in the mouse model of PAH, thereby improving vascular remodeling and angiogenesis. This represents a novel potential therapeutic approach for human pulmonary hypertension [45].
The serum level of miR-30a was found to be elevated in patients with pulmonary hypertension compared to the healthy control group. MiR-30a exhibits predominant expression in the pulmonary arteriole (PA). In animal models of HySu-induced and MCT-induced PAH, genetic knockout of the miR-30a gene effectively reduces right ventricular systolic blood pressure (RVSP), as well as attenuates remodeling of the pulmonary arteriole and hypertrophy of the right ventricle. Furthermore, pharmacological inhibition of miR-30a through intratracheal fluid perfusion (IT-L) administration strategy demonstrates high efficacy [46].
Our previous studies have demonstrated that circ-calm4 promotes pyroptosis in hypoxic PASMCs through the miR-124-3p/PDCD6 pathway [47]. Additionally, this circular RNA also enhances PASMC proliferation via the miR-337-3p/Myo10 pathway [48]. These findings suggest that circ-calm4 may represent a promising therapeutic target for treating pulmonary hypertension.
2.3 MiRNAs in clinical research
In terms of clinical diagnosis, accessing certain tissues such as the cardiac tissue can be challenging. However, miRNAs have emerged as potential biomarkers for disease states and prognostic markers in various diseases. An analysis conducted on plasma samples from 64 untreated patients with pulmonary arterial hypertension, along with 43 patients and healthy controls, demonstrated that miR-636 and miR-187-5p exhibit high diagnostic accuracy in predicting pulmonary hypertension [49]. The findings of these studies indicate that miRNAs have the potential to serve as biomarkers for the diagnosis of PAH.
A significant number of clinical trials involving ncRNAs primarily focus on miRNAs, with over 800 trials conducted, a substantial proportion of which are specifically targeted toward cancer research [50]. Growing evidence suggests that miRNAs play crucial roles in clinical diagnosis and therapy (Figure 2). The first miRNA to transition from basic research to clinical application is miR-122, which has been utilized for the treatment of hepatitis C virus (HCV) [52]. MRX34 represents the pioneering miRNA-based approach for cancer treatment, where it is encapsulated within lipid nanoparticles and administered via intravenous injection into the human body [53]. Furthermore, a nanoparticle formulation named TTX-MC138 has been developed to target anti-miR-10b for breast cancer treatment [54], while an ointment incorporating ionic liquid technology and a mimic of ds-miR-634 has been formulated for skin squamous cell carcinoma (cSCC) therapy [55].
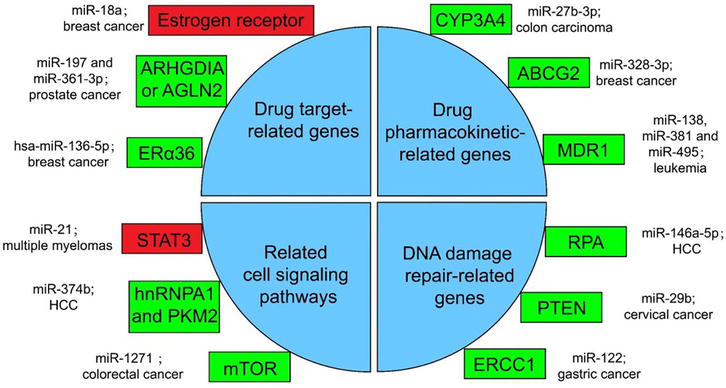
Figure 2.
Several miRNA-related cancer therapy resistance [
The dysregulation of miRNA expression in exosomes has been demonstrated to be a potential therapeutic target for PAH, with the upregulation of miR-21 observed compared to the matched control group [56]. However, there is currently a lack of clinical studies on the therapeutic application of miRNAs in pulmonary hypertension. The future holds immense potential for nucleic acid therapy of pulmonary hypertension, as the combination of meticulous selection of miRNAs from fundamental research and efficient delivery routes will pave the way for groundbreaking advancements in this field.
3. Circular RNAs
3.1 Introduction
Circular RNAs (circRNAs) are a newly discovered class of ncRNAs that lack a 5′ terminal cap and a 3′ terminal poly(A) tail, instead forming a circular structure through covalent bonds [57, 58]. This unique circular conformation distinguishes circRNAs from conventional linear RNAs.
The categorization of circRNAs is based on their origin, which can be classified into three categories: exonic circRNAs derived from exons, circular intronic RNAs derived from introns, and retained-intron circRNAs composed of both exons and introns [59].
CircRNAs possess diverse biological capabilities, including the regulation of target gene expression and modulation of target gene activity through competitive binding with corresponding miRNAs, thereby influencing disease occurrence and progression. These circRNAs are commonly referred to as competitive endogenous RNAs (ceRNAs), acting as molecular “sponges” [60]. Furthermore, circRNAs interact with RNA-binding proteins [61], regulate mRNA stability [62], and serve as protein molecule sponges, with some circRNAs containing binding sites for one or more RNA-binding proteins [63]. Despite being classified as ncRNAs, a subset of circRNAs can encode peptides that exert regulatory functions through peptide encoding [64]. The findings of circRNAs pave the way for future investigations into circRNA biology, offering a novel perspective on our comprehension of gene expression regulation and disease pathogenesis.
3.2 CircRNAs and PAH
CircRNAs exhibit aberrant expression patterns in various diseases, including cancer, neurological disorders, and immune diseases [65, 66, 67, 68]. Additionally, they play a pivotal role in the pathogenesis of pulmonary hypertension.
CircRNAs exhibit aberrant expression in patients with PAH compared to healthy controls, and are involved in the processes of cell proliferation, angiogenesis, inflammation, and fibrosis in PAH patients. This suggests their potential role in disease progression [69, 70, 71]. The circHIPK3/miR-328-3p/STAT3 axis contributes to the pathogenesis of PAH by promoting proliferation, migration, and angiogenesis of human PAECs. The accelerated involvement of circHIPK3 indicates its potential value as a therapeutic target for treating PAH [72]. Hsa_circNFXL1_009 directly acts as a sponge for hsa-miR-29b-2-5p (miR-29b), positively regulating the expression of voltage-gated potassium (K+) channel subfamily B member 1 (KCNB1) at the mRNA level. Delivery of exogenous hsa_circNFXL1_009 attenuates hypoxia-induced proliferation, resistance to apoptosis, and migration of human PASMCs, suggesting its potential use as a therapeutic target for treating PAH [73].
3.3 CircRNAs in clinical research
CircRNAs have emerged as significant markers and targets for the diagnosis and treatment of numerous diseases owing to their distinctive structure, which effectively shields them from exonuclease degradation, rendering them relatively stable in terms of drug efficacy and biological activity (Figure 3).

Figure 3.
Clinical applications of circRNAs and exosomal circRNAs in tumors, including circRNA extraction, detection, as clinical markers and therapeutic strategies [
The abundant presence and remarkable stability of circRNAs in bodily fluids render them an optimal non-invasive biomarker. The identification of circRNAs in serum or tissues holds the potential for early screening, disease monitoring, and prognosis evaluation.
It has been suggested that serum exosomal circCCDC66 secreted and released by pituitary adenoma cells can serve as a potential prognostic biomarker for monitoring tumor dynamics during treatment [76], which highlights the utility of circRNAs as biomarkers for early disease screening, disease monitoring and prognosis evaluation. Researchers have investigated the distinctive expression pattern of hsa_circ_0003416 in the plasma of pediatric patients with PAH resulting from congenital heart disease (CHD). It was observed that hsa_circ_0003416 exhibited a significant downregulation in children affected by PAH-CHD and demonstrated its potential as a biomarker for diagnosing PAH-CHD [77]. This finding implies that circRNAs could serve as valuable diagnostic markers for PAH in clinical settings.
CircRNAs have been extensively investigated in various diseases. For instance, researchers have observed that Steatohepatitis-associated circRNA ATP5B Regulator (SCAR), localized in mitochondria, exerts inhibitory effects on mitochondrial ROS (mROS) production and fibroblast activation. To explore the therapeutic potential of circRNA SCAR
Our previous study discovered that the circ-calm4/miR-337-3p/Myo10 signaling pathway regulates the proliferation of PASMCs at the molecular level and validated this finding in mice. Administration of serotype 5 adenovirus-associated virus (AAV5) through nasal feeding revealed that silencing circ-calm4 alleviated symptoms associated with pulmonary hypertension [48]. Lihuang Su et al. reported that circ-Ntrk2 acts as a sponge for miR-296-5p and miR-296-5p, which bind to the 3′-untranslated region of transforming growth factor beta 1 (TGF β1) mRNA, thereby attenuating TGF β1 translation. Additionally, knocking down circ-Ntrk2 in mice resulted in improved symptoms of PAH [80]. These studies suggest that circRNAs hold potential as therapeutic targets for treating pulmonary hypertension.
Despite the potential of circRNAs in clinical treatment, there are still several challenges that need to be addressed. Antisense technology can selectively inhibit or degrade oncogenic circRNAs, but most loss-of-function studies have only been conducted on preclinical animal models using RNAi. Currently, no circRNA-targeted treatments have reached clinical trials yet [81]. However, delivery of circRNA expression cassettes can be achieved through encapsulation within lipid and/or polymer nanoparticles that can even target specific organelles like mitochondria [82]. Synthetic approaches have also been developed for producing and delivering preformed circRNAs to target cells using non-viral nanoparticles [83]. Additionally, researchers have synthesized artificial circRNA sponges called “circmiRs”
In summary, the current research on circRNA clinical application is still in its early stages. Its future prospects for development in scientific research and clinical investigations are extensive. Studies are required to validate its efficacy and reliability in clinical diagnosis. There is an urgent necessity to develop and enhance advanced methodologies for the design, synthesis, purification, delivery, and therapeutic applications of
4. Long non-coding RNAs
4.1 Introduction
Long non-coding RNAs (lncRNAs) are characterized by their extensive nucleotide sequences, exceeding 200 nucleotides in length, making them a diverse and intriguing group of RNA molecules. LncRNAs play crucial roles in regulating various cellular processes [90].
The biogenesis of lncRNAs follows mechanisms similar to mRNA, involving transcription by RNA polymerase II, 5′ capping, splicing, and 3′ polyadenylation [91]. However, unlike mRNAs, lncRNAs rarely encode proteins and instead exert their functions through a myriad of intricate molecular mechanisms, including acting as molecular scaffolds [74, 75], guiding the assembly of protein complexes [92], serving as decoys by sequestering regulatory proteins [93], and functioning as enhancer-associated transcripts to modulate gene expression over long genomic distances [94].
One notable characteristic of lncRNAs is their specific expression patterns in different tissues and developmental stages [95]. These dynamic expression profiles reflect their involvement in essential biological processes, including embryonic development, cellular differentiation, and tissue homeostasis maintenance. The dysregulation of lncRNAs has increasingly been associated with various human diseases, highlighting their potential as diagnostic markers and therapeutic targets.
4.2 LncRNAs and PAH
LncRNAs play significant roles in cardiovascular disorders by influencing vascular function, cardiac development, and fibrosis [96, 97, 98, 99, 100]. An increasing body of evidence suggests that lncRNAs serve as crucial epigenetic modifiers in pulmonary hypertension. The dysregulation of lncRNAs may impact processes such as vascular remodeling [101], inflammation [102], and endothelial dysfunction [103], thereby contributing to the narrowing of pulmonary vessels observed in PAH [104].
A research from 11 control individuals and 12 pulmonary arterial hypertension patients suggests that JPX modulates RABEP1 (Rab GTPase-binding effector protein expression through miRNAs (miR-145, miR-146 ac, miR-216a, miR-216b, miR-24, miR-33ab, miR-129-5p), thereby impacting CD8+ T cells, NK cells, eosinophils, and other immune cells, ultimately influencing the immune processes associated with PAH. The finding reveals that lncRNA-mRNA pair (JPX-RABEP1) can serve as a potential biomarker in PAH [105]. The rs619586A > G polymorphism in MALAT1 plays a protective role in susceptibility to PAH within the Chinese Han population. This polymorphism has the potential to serve as a novel biomarker for PAH susceptibility and implicates lncRNAs in the etiology of PAH [106]. A study from 41 blood samples, SNHG12 is identified as a competing endogenous RNA (ceRNA) of CCR7 by sequestering hsa-let-7e-5p, which suggests the potential of CCR7, hsa-let-7e-5p, and SNHG12 as candidate biomarkers for PAH.
Although lncRNAs have been reported as potential biomarkers in exosomes or body fluids in diseases such as cancer, they are still in the developmental stage in the field of pulmonary arterial hypertension, but these studies may shed light on the discovery of biomarkers in pulmonary hypertension. Identification of lncRNAs as potential biomarkers holds promise for disease diagnosis, prognosis, and monitoring. The development of non-invasive diagnostic tools based on lncRNA expression profiles could significantly impact early disease detection and patient management.
A study from our team proves that m6A-induced degradation of FENDRR promotes pyroptosis in human pulmonary artery endothelial cells (HPAECs) by regulating the methylation of the DRP1 promoter, providing a potential novel target for hypoxia-induced pulmonary hypertension (HPAH) therapy. The overexpression of the conserved fragment TFO2 of FENDRR prevents HPAH
4.3 LncRNAs in clinical research
RNAs have demonstrated therapeutic potential primarily through small interfering RNA (siRNA) and microRNA (miRNA) applications [25, 26]. LncRNAs have gained significant attention in clinical research due to their diverse roles in various physiological and pathological processes. Advances in understanding the roles of lncRNAs may pave the way for developing novel therapeutic strategies. Targeting disease-associated lncRNAs could offer new avenues for precision medicine, especially in the treatment of pulmonary hypertension.
Given that lncRNA falls in size between siRNA and mRNA, its delivery vehicle may not differ significantly from those already employed in clinical settings for siRNA and mRNA delivery [109]. Therefore, leveraging existing delivery platforms could facilitate the translation of lncRNA-based therapeutics into clinical practice. However, the intricate secondary structure of lncRNAs poses challenges for their exogenous delivery, hindering the development of lncRNA-based drugs. As a result, instead of directly transferring lncRNAs, many lncRNA-based drugs opt to target lncRNAs to modulate their expression. The next is an exploration of diverse delivery systems utilized for RNA therapeutics, with some having undergone testing for lncRNA and nucleic acid delivery targeting lncRNAs.
Numerous studies have explored the potential of lncRNAs as diagnostic and prognostic biomarkers in various cancers, such as LINC01535 in breast cancer [110], MLETA1 in lung cancer [111], and HCG18 in cholangiocarcinoma [112]. LncRNAs ANRIL and HOTAIR can be used as biomarkers for cardiovascular disease risk prediction [113]. LncRNA PCA3 is highly expressed in prostate cancer and is considered useful for prostate cancer diagnosis [112]. However, lncRNAs as PAH biomarkers are still being researched.
5. PIWI-interacting RNA
5.1 Introduction
PiRNAs (PIWI-interacting RNA) are a class of small non-coding RNA molecules, typically ranging from 24 to 32 nucleotides in length [114]. PiRNAs exhibit highly conserved and diverse sequences. Unlike other types of small RNAs such as miRNAs and siRNAs, piRNAs are primarily generated through the interaction between transposon factors and the genome [115]. PiRNAs are mainly expressed in the reproductive system. They form complexes with PIWI proteins to form piRNA-induced silencing complexes (piRISCs) that play crucial roles in protecting genome stability during transposition, suppressing transposon activity, and regulating gene expression and epigenetic modifications [116]. The initial belief that piRNAs are exclusively involved in germ cell development has been challenged by recent research, which has demonstrated their expression in various tissues and cell types. Furthermore, these findings highlight the involvement of piRNAs in maintaining immune homeostasis and underscore their significance in diseases such as cancer [117].
5.2 PiRNAs and PAH
The involvement of piRNAs in crucial processes such as cardiovascular remodeling, inflammatory response, and cell proliferation is closely associated with the pathophysiological mechanisms underlying pulmonary arterial hypertension. PiRNA-63076 is upregulated in hypoxic PASMCs and the lung tissues of hypoxia-induced mice, which promotes the proliferation of PASMCs by regulating the interaction of acyl-CoA dehydrogenase (Acadm) with Piwi protein. PiRNA-63076 partially inhibits the expression of Acadm by mediating DNA methylation in the promoter region of Acadm, providing a new perspective on understanding the pathophysiological process of pulmonary arterial hypertension [118]. Some piRNAs from the extracellular vesicles of PAH patients exhibited different expression patterns correlated with the severity of PAH, indicating a potential role for piRNAs in the development and progression of PAH [119].
5.3 PiRNAs in clinical research
The expression of piRNAs demonstrates disease-specificity, rendering them potential diagnostic biomarkers. Researchers have already employed piRNAs for early tumor detection and prediction of patient prognosis, showcasing promising clinical applicability [120]. Assessing piRNAs levels in blood, urine, or other bodily fluids may be feasible to aid in the early diagnosis, classification, and prognostic evaluation of diseases.
PiRNAs also play a role in the treatment of diseases. PiRNA-54265 is upregulated in colorectal cancer. AntagopiR54265, the inhibitor of piRNA-54265, significantly suppressed the growth and metastasis of implanted tumors [121]. By targeting piRNAs as therapeutic targets, the regulation of relevant gene expression can be achieved through interference with specific interactions between piRNAs and Piwi proteins. Through the design and synthesis of specific piRNA ligands, it is possible to precisely modulate gene expression, signal pathways, and cellular processes, thereby intervening in disease development.
The research on PiRNAs is still in its nascent stage. The clinical application of piRNAs is still in the research stages, requiring further experiments and clinical studies to validate its accuracy, feasibility, and safety. PiRNAs hold promise as diagnostic biomarkers for various diseases, a powerful tool for targeted therapy, and a strategic approach toward cell therapy and regenerative medicine [120].
6. Small nucleolar RNA
6.1 Introduction
SnoRNAs (small nucleolar RNA) are a class of small non-coding RNA molecules that mainly exist in the nucleolus. They play regulatory roles in the nucleolus and are involved in the modification and maturation processes of ribosomal RNAs (rRNAs) [122]. SnoRNAs are typically composed of 60–300 nucleotides and are divided into two main categories: C/D box snoRNAs and H/ACA box snoRNAs. C/D box snoRNAs mainly form nucleotide methyltransferase complexes with proteins to carry out 2′-O-methylation modifications at specific positions in rRNAs. H/ACA box snoRNAs form complexes with proteins to maintain RNA stability and localization and guide post-transcriptional modifications in rRNAs [123].
SnoRNAs are involved in various cellular processes, such as proliferation, transcription, and RNA splicing, which are associated with tumor development and metastasis [124]. The abnormal expression or dysfunction of snoRNAs may play significant roles in the development of diseases, including cardiovascular diseases, congenital disorders, and genetic diseases [125].
6.2 SnoRNAs and PAH
A study from different types of pulmonary arterial hypertension patients (including idiopathic pulmonary arterial hypertension (IPAH), connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH), and congenital heart disease-associated pulmonary arterial hypertension (CHD-PAH)) shows that there are 30 differentially expressed snoRNAs in lung tissues [126]. SNORD20, SCARNA4, and SNORD94 exhibit consistent differential expression in all three PAH, suggesting the three snoRNAs play important roles in the pathophysiological processes of PAH [126].
The relationship between snoRNAs and diseases still encompasses numerous unexplored areas. Further investigations are imperative to accumulate additional evidence elucidating the association between snoRNAs and diseases, thereby offering novel insights and approaches for disease prevention and therapy.
7. tRNA-derived small RNA
7.1 Introduction
TsRNA (tRNA-derived small RNA) refers to a class of small RNAs generated from tRNAs. TRNAs are a common type of RNA that primarily transports amino acids to the ribosome during protein synthesis. However, recent research has discovered that tRNAs participate in other cellular processes through various mechanisms and give rise to various tsRNAs [127]. The generation of tsRNAs mainly involves two mechanisms: tRNAs cleavage and modification and the generation of cleavage products through non-canonical functions of tRNAs. TsRNAs have diverse biological functions, including regulation of gene expression, translational control, and impact on cellular processes such as growth, division, and apoptosis [128]. Abnormal expression of tsRNAs may be associated with the occurrence and development of tumors. For example, tsRNAs are significantly increased or decreased in cancer cells, which may affect the expression of tumor suppressor genes and promote the growth and spread of tumor cells [129].
7.2 tsRNAs and PAH
An increasing number of studies are investigating the association of tsRNAs with cardiovascular diseases, such as pulmonary hypertension [32, 97, 99]. Altered expression of tsRNAs in the blood and lung tissue of PAH patients has been found. A study conducted a small RNA microarray analysis of tsRNAs in the plasma of pulmonary arterial hypertension patients and a healthy control group identified a total of 816 tsRNAs significantly regulated in PAH, with 243 upregulated and 573 downregulated [130]. Gene Ontology analysis showed that these tsRNAs might be involved in cellular metabolic conditions and other processes, suggesting that tsRNAs could potentially serve as novel biomarkers and therapeutic targets in idiopathic PAH [130].
8. Delivery tools for RNAs
Gene therapy is extensively utilized for the treatment of incurable disorders and has become a routine procedure in clinical practice. The main challenge in the development and application of RNA drugs lies in their delivery technology, as opposed to small molecule and protein drugs. Unlike these counterparts, RNA molecules are negatively charged, sensitive to ubiquitous RNases, and primarily act intracellularly. Therefore, the advancement of delivery systems is crucial for addressing existing issues

Figure 4.
Schematic overview of administration routes and features of miRNA therapeutics [
8.1 Viral vector for RNA delivery
Viral vectors have emerged as indispensable components of gene therapy due to their inherent ability to infect mammalian cells and effectively deliver genetic material [132]. Adeno-associated virus (AAV), adenovirus (AdVs), and lentiviral vectors (LVV) are increasingly being recognized as fundamental therapeutic delivery systems owing to their usability and safety profile [133].
However, the utilization of viral vectors can give rise to two types of adverse effects: a response triggered by the virus’s entry or a reaction induced by the delivered genetic material. Any viral-based gene therapy approach necessitates thoroughly evaluating potential side effects and safety concerns.
AAV is a small, non-enveloped virus requiring helper viruses such as AdV, herpesvirus, and some others for replication. AAV is a natural human symbiont, and a weak immune response makes it a suitable vector for gene therapy [134, 135, 136].
AdV, a non-enveloped virus, contains protein-coding genes belonging to Adenoviridae [137, 138]. The wide applications of AdV are due to their high levels of gene expression, transduction efficiency, and ability to deliver transgenes of up to 8 kbp in size [139, 140].
Lentivirus is an enveloped virus belonging to the family Retroviridae. The advantages of LVVs are the packaging capacity of up to 10 kb [141], low cytotoxicity, and lack of viral replication after transduction.
8.2 Non-viral vectors for RNA delivery
The limitations of viral vector delivery systems such as immunogenicity and mutagenicity restrict their clinical application and prompt the development of non-viral gene carriers using different materials to achieve successful

Figure 5.
A schematic representation of some nanocarriers used for miRNA delivery [
8.2.1 Nanoparticles
8.2.1.1 Lipid nanoparticles (LNP)
LNPs stand as the sole FDA-approved carrier for macromolecular nucleic acid drugs, facilitating enhancements in clinical trials [143, 144]. Three LNP-based RNA drugs have been approved on the market, including Alnylam’s Patisiran (ONPATTRO™), Pfizer’s BNT162b2, and Moderna’s mRNA-1273. Despite LNPs exhibiting lower transfection efficiency compared to lentiviral vectors, their advanced, safe, relatively convenient, and cost-effective construction process renders them practical alternatives (Figure 6) [145].

Figure 6.
Illustration of targeted therapy using sophisticated RNA-LNPs designed to target specific tissues and organs [
8.2.1.2 GalNAc
GalNAc is a high-affinity targeting ligand of the Asialoglycoprotein receptor (ASGPR) [146]. When GalNAc binds to ASGPR, it can enter the endosome through clathrin-mediated endocytosis. Then, ASGPR dissociates from GalNAc-siRNA conjugate and circulates back to the surface of cells. The delivery strategy based on GalNAc offers more advantages compared to LNP due to its subcutaneous administration capability and ease of scaling up. Currently, a significant number of RNA drugs in clinical trials are utilizing GalNAc technology.
8.2.1.3 Lipid-based nanoparticles (LBNPs)
Lipid-based nanoparticles (LBNPs) encompass liposomes, solid lipid nanoparticles (SLN), and nanostructured lipid carriers (NLC). These nanoparticles possess the ability to transport both hydrophobic and hydrophilic molecules, exhibit minimal or no toxicity, and prolong drug action duration [147]. The advantages of LBNPs include excellent temporal and thermal stability, high loading capacity, ease of preparation, low production costs, and suitability for large-scale industrial production due to their natural source origin. LBNPs have been extensively evaluated
8.2.1.4 Polymeric nanoparticles
Polymeric nanoparticles (NPs) are particles ranging in size from 1 to 1000 nm and can be loaded with active compounds entrapped within or surface-adsorbed onto the polymeric core. Polymeric NPs have garnered significant interest due to their unique properties resulting from their small size [149, 150, 151]. As drug carriers, polymeric NPs can control release, protect drugs and other biologically active molecules against environmental factors, as well as improve bioavailability and therapeutic index [151, 152].
8.2.1.5 Aptamer
Aptamer is a single-strand oligonucleotide that can bind specifically to the target molecule, which forms a specific three-dimensional structure after adaptive folding through various interaction forces such as nucleotide base complementary pairing. This binding is comparable to that of monoclonal antibodies. Compared with antibodies, aptamers have many advantages, such as a wider range of target molecules, better thermal stability, smaller molecular weight, chemical synthesis, small batch differences, easy modification, and so on. Aptamers allowed targeted delivery of RNA therapeutics through cell-specific surface markers, and once inside the cell, the nanoparticles induced lysosomal leakage that released the RNA oligonucleotides into the cytosol to achieve gene silencing [153].
8.2.1.6 Biomimetic nanomaterials
Biomimetic nanomaterials are an intersection between traditional synthetic nanomedicine and biotechnologies, offering biocompatibility, safety, and scalability of formulations with complex behaviors. The composition of biomimetic nanomaterials involves the integration of synthetic nanomaterials with simplified designs and essential biological components, which are crucial for achieving the desired effect. This approach enables enhanced biocompatibility and scalability in production [154]. Among the various biomimetic approaches investigated for enhancing the pharmacokinetics of nanoparticles (NPs), cell membranes and their constituents have emerged as a captivating opportunity [155].
8.2.2 Exosomes
Exosomes are small vesicles released by cells, typically with diameters ranging from 40 to 160 nm, which elicit minimal immune responses and possess an ability to traverse biological barriers such as cellular membranes and the blood-brain barrier [156, 157]. Exosomes contain a diverse array of biomolecules, including proteins, lipids, nucleic acids, and cell surface receptors [158, 159] and protect these molecules from degradation and facilitate intercellular communication across different tissues within the organism [82]. The challenges of utilization of exosomes as carriers for RNA delivery are how to enable large-scale production and ensure consistent quality of exosomes [160, 161]
8.2.3 Hydrogels
Macroscopic hydrogels are soft and water-swollen, which possess remarkable features such as biodegradability, tunable physiochemical properties, and injectability (Figure 7). Hydrogels have attracted enormous attention for use in RNA therapy recently. RNA can be loaded into hydrogels either by direct inclusion of the naked RNA or by encapsulating RNA nanocarriers. Hydrogels could improve RNA stability, reduce unnecessary loss of therapeutics during systemic delivery, mitigate undesirable off-target toxicities, and avoid the necessity of multiple doses [162].

Figure 7.
Advantages of hydrogels as a platform for RNA delivery [
8.2.4 Protein/peptide
A protein derived from retrovirus is found to transport RNAs outside the cell in virus-like particles (VLPs). This selective endogenous encapsidation for cellular delivery enables the delivery of exogenous mRNA cargos, such as Cre and Cas9, into cells
8.2.5 Virus-like particles (VLPs)
VLPs possess protein coats resembling viruses, which are capable of adapting to fluctuations in PAH and temperature. Their surface can be customized with various peptides to target distinct cell types (Figure 8) [165].

Figure 8.
Delivery of mRNA cargo by virus-like particles derived from endogenous retroelements [
9. Molecular docking in RNA-based therapeutics
Molecular docking is a key tool in structural molecular biology and computer-assisted drug design. The successful docking methods effectively explore high-dimensional spaces and employ a scoring function that accurately ranks candidate dockings. Docking can be utilized for virtual screening of extensive compound libraries, ranking the outcomes, and proposing structural hypotheses on how RNAs inhibit targets, which is invaluable in lead optimization [166].
The rapidly expanding therapeutic interest in RNA-targeted drug discovery has led to an increasing demand for computational tools that can predict interactions between RNA and ligands. Virtual screening remains a crucial initial step in the design of novel drugs, particularly when only information about the targeted RNA is available. Artificial intelligence (AI) is often portrayed as a new Industrial Revolution [167]. AI finds applications in various domains, including molecular simulation for drug discovery [17]. Various methods for docking (sampling) and scoring have been developed to expedite this process, thereby enhancing our understanding of the mechanisms underlying RNA-ligand binding. Physics-based and knowledge-based approaches have demonstrated promising success in predicting both ligand-binding poses and affinities [168]. The process of molecular docking verification, see Figure 9.

Figure 9.
The process of molecular docking verification.
It has been demonstrated that neglecting the flexibility of the protein (receptor) during protein-ligand docking can lead to inaccurate predictions of binding modes [169]. This issue becomes even more critical when dealing with ligand binding to RNA, which exhibits greater structural flexibility compared to proteins. RNA-Ligand DOCKing (RLDOCK) is a recently developed docking model for flexible ligands using a multiconformer approach [170, 171].
Successful computational drug discovery requires the integration of three essential components: (1) a methodology for identifying druggable RNA targets, (2) a computationally efficient sampling algorithm for exploring RNA conformations, ligand conformers, and ligand binding poses, and (3) accurate scoring functions to evaluate the structures of RNA-ligand complexes and assess their binding affinity [168].
Due to the limitations of the delivery system, it is currently restricted to small RNA delivery. However, emerging evidence highlights the crucial role of lncRNAs and circRNAs in disease pathogenesis. Given their extended length, current delivery systems are inadequate for introducing these RNAs into the body. Molecular docking analysis between RNA and target molecules enables the identification of active domains and nucleotide sites that exert local key functions. Consequently, short functional RNA sequences can be designed for these regions and introduced into the body using an appropriate delivery system. Upon demonstrating efficacy at cellular and animal levels, this approach holds promise for developing vaccines or nucleic acid therapeutics suitable for clinical applications.
10. Prospects
In order to improve the targeting and efficacy of RNA therapeutics, improving RNA stability, reducing RNA-induced immunological reactions, and improving the efficiency of RNA delivery systems are the main challenges facing the development of RNA drugs in the future. The integration of ncRNA identification against conserved regions within key genomic sites associated with PAH, coupled with the development of safe nanomedicines and the production of dry powders for inhalation to enhance storage stability, positions us favorably in our pursuit of RNA therapeutics.
Using computer-aided molecular docking technology to predict the interaction and affinity of ncRNAs with their targets through 3D structure, it is helpful to construct ncRNA drugs based on the finding of effective domains of ncRNAs. Then, the constructed RNA will play therapeutic roles through suitable in vivo delivery systems. With the development of various computational tools developed for RNA-targeted drug discovery, a CASP- and D3R-like [172, 173] community-wide events with blind tests and well-curated benchmark datasets, similar to those the benchmarks widely used in the protein-ligand modeling community, would be much needed [67, 174, 175, 176, 177, 178].
In addition, the discovery of specific markers for various cells and specific lipid components that can target the delivery of RNA-LNPs is crucial to improving the targeting effect of RNA delivery systems. The conjugation and expression of specific peptides or aptamers on RNA-LNPs are likely to be the most reliable strategy to recognize and deliver RNA-LNPs into specific organs and/or cells. In the future, advances in basic research will make it possible to achieve the precision and efficiency of ncRNA therapies.
Moreover, it is imperative to establish a correlation between the outcomes of our
In conclusion, the roles of ncRNAs in PAH have been continuously clarified. Treating PAH patients with ncRNAs through appropriate delivery systems will be the trend of PAH therapy in the future. The application of molecular docking technology will help ncRNAs play a targeted therapeutic role in clinical medicine.
References
- 1.
Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Kardiologia Polska. 2015; 73 (12):1127-1206 - 2.
Weber L, Rickli H, Haager PK, et al. Haemodynamic mechanisms and long-term prognostic impact of pulmonary hypertension in patients with severe aortic stenosis undergoing valve replacement. European Journal of Heart Failure. 2019; 21 (2):172-181 - 3.
Bourgeois A, Lambert C, Habbout K, et al. FOXM1 promotes pulmonary artery smooth muscle cell expansion in pulmonary arterial hypertension. Journal of Molecular Medicine (Berlin, Germany). 2018; 96 (2):223-235 - 4.
Bourgeois A, Omura J, Habbout K, Bonnet S, Boucherat O. Pulmonary arterial hypertension: New pathophysiological insights and emerging therapeutic targets. The International Journal of Biochemistry & Cell Biology. 2018; 104 :9-13 - 5.
Chelladurai P, Seeger W, Pullamsetti SS. Epigenetic mechanisms in pulmonary arterial hypertension: The need for global perspectives. European Respiratory Review. 2016; 25 (140):135-140 - 6.
Veith C, Schermuly RT, Brandes RP, Weissmann N. Molecular mechanisms of hypoxia-inducible factor-induced pulmonary arterial smooth muscle cell alterations in pulmonary hypertension. The Journal of Physiology. 2016; 594 (5):1167-1177 - 7.
Wakiyama M, Yokoyama S. MicroRNA-mediated deadenylation in a mammalian cell-free system. Methods in Molecular Biology. 2014; 1125 :341-351 - 8.
Xiao L, Wang J, Ju S, Cui M, Jing R. Disorders and roles of tsRNA, snoRNA, snRNA and piRNA in cancer. Journal of Medical Genetics. 2022; 59 (7):623-631 - 9.
Botti G, Marra L, Malzone MG, et al. LncRNA HOTAIR as prognostic circulating marker and potential therapeutic target in patients with tumor diseases. Current Drug Targets. 2017; 18 (1):27-34 - 10.
Di X, Jin X, Li R, Zhao M, Wang K. CircRNAs and lung cancer: Biomarkers and master regulators. Life Sciences. 2019; 220 :177-185 - 11.
Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M. Deciphering miRNAs' action through miRNA editing. International Journal of Molecular Sciences. 2019; 20 (24):6249 - 12.
Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-targeted therapeutics. Cell Metabolism. 2018; 27 (4):714-739 - 13.
Yin W, Rogge M. Targeting RNA: A transformative therapeutic strategy. Clinical and Translational Science. 2019; 12 (2):98-112 - 14.
Yu AM, Jian C, Yu AH, Tu MJ. RNA therapy: Are we using the right molecules? Pharmacology & Therapeutics. 2019; 196 :91-104 - 15.
Yu AM, Choi YH, Tu MJ. RNA drugs and RNA targets for small molecules: Principles, progress, and challenges. Pharmacological Reviews. 2020; 72 (4):862-898 - 16.
Connelly C, Moon M, Schneekloth J. The emerging role of RNA as a therapeutic target for small molecules. Cell Chemical Biology. 2016; 23 (9):1077-1090 - 17.
Somody JC, MacKinnon SS, Windemuth A. Structural coverage of the proteome for pharmaceutical applications. Drug Discovery Today. 2017; 22 (12):1792-1799 - 18.
Warner KD, Hajdin CE, Weeks KM. Principles for targeting RNA with drug-like small molecules. Nature Reviews Drug Discovery. 2018; 17 (8):547-558 - 19.
Tessaro F, Scapozza L. How ‘protein-docking’ translates into the new emerging field of docking small molecules to nucleic acids? Molecules. 2020; 25 (12):2749-2764 - 20.
Costales MG, Childs-Disney JL, Haniff HS, Disney MD. How we think about targeting RNA with small molecules. Journal of Medicinal Chemistry. 2020; 63 (17):8880-8900 - 21.
Clamp M, Fry B, Kamal M, Xie X, Cuff J, Lin MF, et al. Distinguishing protein-coding and noncoding genes in the human genome. Proceedings of the National Academy of Sciences. 2007; 104 (49):19428-19433 - 22.
Hopkins AL, Groom CR. The druggable genome. Nature Reviews Drug Discovery. 2002; 1 (9):727-730 - 23.
Dixon SJ, Stockwell BR. Identifying druggable disease-modifying gene products. Current Opinion in Chemical Biology. 2009; 13 (5):549-555 - 24.
Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nature Reviews Drug Discovery. 2006; 5 (12):993-996 - 25.
Jordan PC, Stevens SK, Deval J. Nucleosides for the treatment of respiratory RNA virus infections. Antiviral Chemistry & Chemotherapy. 2018; 26 :2040206618764483 - 26.
Sullenger BA, Nair S. From the RNA world to the clinic. Science. 2016; 352 (6292):1417-1420 - 27.
Dorsett Y, Tuschl T. siRNAs: Applications in functional genomics and potential as therapeutics. Nature Reviews. Drug Discovery. 2004; 3 (4):318-329 - 28.
Kifle ZD, Ayele AG, Enyew EF. Drug repurposing approach, potential drugs, and novel drug targets for COVID-19 treatment. Journal of Environmental and Public Health. 2021; 2021 :6631721 - 29.
Liu C, Zhou Q , Li Y, et al. Research and Development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Central Science. 2020; 6 (3):315-331 - 30.
Rothman AM, Arnold ND, Pickworth JA, et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. The Journal of Clinical Investigation. 2016; 126 (7):2495-2508 - 31.
Düzgün Z, Kayıkçıoğlu LM, Aktan Ç, et al. Decreased circulating microRNA-21 and microRNA-143 are associated to pulmonary hypertension. Turkish Journal of Medical Sciences. 2023; 53 (1):130-141 - 32.
Wang F, Zuroske T, Watts JK. RNA therapeutics on the rise. Nature Reviews. Drug Discovery. 2020; 19 (7):441-442 - 33.
Roberts TC, Langer R, Wood M. Advances in oligonucleotide drug delivery. Nature Reviews. Drug Discovery. 2020; 19 (10):673-694 - 34.
Kulkarni JA, Witzigmann D, Thomson SB, et al. The current landscape of nucleic acid therapeutics. Nature Nanotechnology. 2021; 16 (6):630-643 - 35.
Merkel OM, Librizzi D, Pfestroff A, et al. Stability of siRNA polyplexes from poly(ethylenimine) and poly(ethylenimine)-g-poly(ethylene glycol) under in vivo conditions: Effects on pharmacokinetics and biodistribution measured by Fluorescence Fluctuation Spectroscopy and Single Photon Emission Computed Tomography (SPECT) imaging. Journal of Controlled Release. 2009;138 (2):148-159 - 36.
Titze-de-Almeida R, David C, Titze-de-Almeida SS. The race of 10 synthetic RNAi-based drugs to the pharmaceutical market. Pharmaceutical Research. 2017; 34 (7):1339-1363 - 37.
Mehta A, Michler T, Merkel OM. siRNA therapeutics against respiratory viral infections-what have we learned for potential COVID-19 therapies. Advanced Healthcare Materials. 2021; 10 (7):e2001650 - 38.
Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nature Cell Biology. 2009; 11 (3):228-234 - 39.
Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nature Reviews. Drug Discovery. 2013; 12 (11):847-865 - 40.
Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: Approaches and considerations. Nature Reviews. Genetics. 2012; 13 (5):358-369 - 41.
Gu S, Jin L, Zhang F, Sarnow P, Kay MA. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nature Structural & Molecular Biology. 2009; 16 (2):144-150 - 42.
Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ. 2018; 360 :j5492 - 43.
Bertero T, Lu Y, Annis S, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. The Journal of Clinical Investigation. 2014; 124 (8):3514-3528 - 44.
Chen T, Zhou G, Zhou Q , et al. Loss of microRNA-17∼92 in smooth muscle cells attenuates experimental pulmonary hypertension via induction of PDZ and LIM domain 5. American Journal of Respiratory and Critical Care Medicine. 2015; 191 (6):678-692 - 45.
Fu J, Bai P, Chen Y, Yu T, Li F. Inhibition of miR-495 improves both vascular Remodeling and angiogenesis in pulmonary hypertension. Journal of Vascular Research. 2019; 56 (2):97-106 - 46.
Ma W, Qiu Z, Bai Z, et al. Inhibition of microRNA-30a alleviates vascular remodeling in pulmonary arterial hypertension. Molecular Therapy - Nucleic Acids. 2021; 26 :678-693 - 47.
Jiang Y, Liu H, Yu H, et al. Circular RNA Calm4 regulates hypoxia-induced pulmonary arterial smooth muscle cells Pyroptosis via the Circ-Calm4/miR-124-3p/PDCD6 Axis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2021; 41 (5):1675-1693 - 48.
Zhang J, Li Y, Qi J, et al. Circ-calm4 serves as an miR-337-3p sponge to regulate Myo10 (myosin 10) and promote pulmonary artery smooth muscle proliferation. Hypertension. 2020; 75 (3):668-679 - 49.
Errington N, Iremonger J, Pickworth JA, et al. A diagnostic miRNA signature for pulmonary arterial hypertension using a consensus machine learning approach. eBioMedicine. 2021; 69 :103444 - 50.
Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell. 2019; 179 (5):1033-1055 - 51.
Prakash S, Dhanushkodi NR, Zayou L, et al. Cross-protection induced by highly conserved human B, CD4+, and CD8+ T cell epitopes-based coronavirus vaccine against severe infection, disease, and death caused by multiple SARS-CoV-2 variants of concern. bioRxiv. 24 May 2023. 541850 [Preprint] - 52.
Hu J, Xu Y, Hao J, Wang S, Li C, Meng S. MiR-122 in hepatic function and liver diseases. Protein & Cell. 2012; 3 (5):364-371 - 53.
Zhang L, Liao Y, Tang L. MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. Journal of Experimental & Clinical Cancer Research. 2019; 38 (1):53 - 54.
Parrella P, Barbano R, Pasculli B, et al. Evaluation of microRNA-10b prognostic significance in a prospective cohort of breast cancer patients. Molecular Cancer. 2014; 13 :142 - 55.
Inoue J, Inazawa J. Cancer-associated miRNAs and their therapeutic potential. Journal of Human Genetics. 2021; 66 (9):937-945 - 56.
Chang WT, Lee WC, Lin YW, et al. Transpulmonary expression of exosomal microRNAs in idiopathic and congenital heart disease-related pulmonary arterial hypertension. Journal of the American Heart Association. 2023; 12 (23):e031435 - 57.
Ali MK, Schimmel K, Zhao L, et al. The role of circular RNAs in pulmonary hypertension. The European Respiratory Journal. 2022; 60 (6):2200012 - 58.
Yu CY, Kuo HC. The emerging roles and functions of circular RNAs and their generation. Journal of Biomedical Science. 2019; 26 (1):29 - 59.
Li J, Xu Q , Huang ZJ, et al. CircRNAs: A new target for the diagnosis and treatment of digestive system neoplasms. Cell Death & Disease. 2021; 12 (2):205 - 60.
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: The Rosetta stone of a hidden RNA language. Cell. 2011; 146 (3):353-358 - 61.
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Research. 2016; 44 (6):2846-2858 - 62.
Legnini I, Di Timoteo G, Rossi F, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Molecular Cell. 2017; 66 (1):22-37.e9 - 63.
Conn SJ, Pillman KA, Toubia J, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015; 160 (6):1125-1134 - 64.
Pan Z, Cai J, Lin J, et al. A novel protein encoded by circFNDC3B inhibits tumor progression and EMT through regulating snail in colon cancer. Molecular Cancer. 2020; 19 (1):71 - 65.
Hanan M, Soreq H, Kadener S. CircRNAs in the brain. RNA Biology. 2017; 14 (8):1028-1034 - 66.
Kristensen LS, Hansen TB, Venø MT, Kjems J. Circular RNAs in cancer: Opportunities and challenges in the field. Oncogene. 2018; 37 (5):555-565 - 67.
Li H, Li K, Lai W, et al. Comprehensive circular RNA profiles in plasma reveals that circular RNAs can be used as novel biomarkers for systemic lupus erythematosus. Clinica Chimica Acta. 2018; 480 :17-25 - 68.
Vo JN, Cieslik M, Zhang Y, et al. The landscape of circular RNA in cancer. Cell. 2019; 176 (4):869-881.e13 - 69.
Chouvarine P, Photiadis J, Cesnjevar R, et al. RNA expression profiles and regulatory networks in human right ventricular hypertrophy due to high pressure load. iScience. 2021; 24 (3):102232 - 70.
Guo HM, Liu ZP. Up-regulation of circRNA_0068481 promotes right ventricular hypertrophy in PAH patients via regulating miR-646/miR-570/miR-885. Journal of Cellular and Molecular Medicine. 2021; 25 (8):3735-3743 - 71.
Miao R, Wang Y, Wan J, et al. Microarray expression profile of circular RNAs in chronic thromboembolic pulmonary hypertension. Medicine (Baltimore). 2017; 96 (27):e7354 - 72.
Hong L, Ma X, Liu J, et al. Circular RNA-HIPK3 regulates human pulmonary artery endothelial cells function and vessel growth by regulating microRNA-328-3p/STAT3 axis. Pulmonary Circulation. 2021; 11 (2):20458940211000234 - 73.
Jin X, Xu Y, Guo M, et al. hsa_circNFXL1_009 modulates apoptosis, proliferation, migration, and potassium channel activation in pulmonary hypertension. Molecular Therapy - Nucleic Acids. 2021; 23 :1007-1019 - 74.
Zhang F, Jiang J, Qian H, Yan Y, Xu W. Exosomal circRNA: Emerging insights into cancer progression and clinical application potential. Journal of Hematology & Oncology. 2023; 16 (1):67 - 75.
Zhang W, Zhao J, Deng L, et al. INKILN is a novel long noncoding RNA promoting vascular smooth muscle inflammation via scaffolding MKL1 and USP10. Circulation. 2023; 148 (1):47-67 - 76.
Yue X, Lan F, Liu W. Serum exosomal circCCDC66 as a potential diagnostic and prognostic biomarker for pituitary adenomas. Frontiers in Oncology. 2023; 13 :1268778 - 77.
Huang Y, Su D, Ye B, et al. Expression and clinical significance of circular RNA hsa_circ_0003416 in pediatric pulmonary arterial hypertension associated with congenital heart disease. Journal of Clinical Laboratory Analysis. 2022; 36 (4):e24273 - 78.
Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. Journal of Controlled Release. 2020; 318 :1-15 - 79.
Fan Y, Wang J, Jin W, et al. CircNR3C2 promotes HRD1-mediated tumor-suppressive effect via sponging miR-513a-3p in triple-negative breast cancer. Molecular Cancer. 2021; 20 (1):25 - 80.
Su L, Li X, Mao X, et al. Circ-Ntrk2 acts as a miR-296-5p sponge to activate the TGF-β1/p38 MAPK pathway and promote pulmonary hypertension and vascular remodelling. Respiratory Research. 2023; 24 (1):78 - 81.
Kristensen LS, Jakobsen T, Hager H, Kjems J. The emerging roles of circRNAs in cancer and oncology. Nature Reviews. Clinical Oncology. 2022; 19 (3):188-206 - 82.
Zhao Q , Liu J, Deng H, et al. Targeting mitochondria-located circRNA SCAR alleviates NASH via reducing mROS output. Cell. 2020; 183 (1):76-93.e22 - 83.
Wesselhoeft RA, Kowalski PS, Parker-Hale FC, Huang Y, Bisaria N, Anderson DG. RNA circularization diminishes immunogenicity and can extend translation duration In vivo. Molecular Cell. 2019; 74 (3):508-520.e4 - 84.
Lavenniah A, Luu T, Li YP, et al. Engineered circular RNA sponges act as miRNA inhibitors to attenuate pressure overload-induced cardiac hypertrophy. Molecular Therapy. 2020; 28 (6):1506-1517 - 85.
Liu X, Abraham JM, Cheng Y, et al. Synthetic circular RNA functions as a miR-21 sponge to suppress gastric carcinoma cell proliferation. Molecular Therapy - Nucleic Acids. 2018; 13 :312-321 - 86.
Schreiner S, Didio A, Hung LH, Bindereif A. Design and application of circular RNAs with protein-sponge function. Nucleic Acids Research. 2020; 48 (21):12326-12335 - 87.
Wang Z, Ma K, Cheng Y, et al. Synthetic circular multi-miR sponge simultaneously inhibits miR-21 and miR-93 in esophageal carcinoma. Laboratory Investigation. 2019; 99 (10):1442-1453 - 88.
Qu L, Yi Z, Shen Y, et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell. 2022; 185 (10):1728-1744.e16 - 89.
Li H, Peng K, Yang K, et al. Circular RNA cancer vaccines drive immunity in hard-to-treat malignancies. Theranostics. 2022; 12 (14):6422-6436 - 90.
Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009; 136 (4):629-641 - 91.
Wierzbicki AT, Haag JR, Pikaard CS. Noncoding transcription by RNA polymerase pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell. 2008; 135 (4):635-648 - 92.
Malakar P, Shilo A, Mogilevsky A, et al. Long noncoding RNA MALAT1 promotes hepatocellular carcinoma development by SRSF1 upregulation and mTOR activation. Cancer Research. 2017; 77 (5):1155-1167 - 93.
Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annual Review of Biochemistry. 2012; 81 :145-166 - 94.
Ørom UA, Shiekhattar R. Long noncoding RNAs usher in a new era in the biology of enhancers. Cell. 2013; 154 (6):1190-1193 - 95.
Eptaminitaki GC, Stellas D, Bonavida B, Baritaki S. Long non-coding RNAs (lncRNAs) signaling in cancer chemoresistance: From prediction to druggability. Drug Resistance Updates. 2022; 65 :100866 - 96.
Omura J, Habbout K, Shimauchi T, et al. Identification of long noncoding RNA H19 as a new biomarker and therapeutic target in right ventricular failure in pulmonary arterial hypertension. Circulation. 2020; 142 (15):1464-1484 - 97.
Wang S, Luo Z, Yuan L, et al. tRNA-derived small RNAs: Novel insights into the pathogenesis and treatment of cardiovascular diseases. Journal of Cardiovascular Translational Research. 2023; 16 (2):300-309 - 98.
Wang T, Sung TC, Yu T, et al. Next-generation materials for RNA-lipid nanoparticles: Lyophilization and targeted transfection. Journal of Materials Chemistry B. 2023; 11 (23):5083-5093 - 99.
Wang Y, Chen D, Xie H, et al. LncRNA GAS5 suppresses TGF-β1-induced transformation of pulmonary pericytes into myofibroblasts by recruiting KDM5B and promoting H3K4me2/3 demethylation of the PDGFRα/β promoter. Molecular Medicine. 2023; 29 (1):32 - 100.
Yu J, Wang W, Yang J, et al. LncRNA PSR regulates vascular remodeling through encoding a novel protein Arteridin. Circulation Research. 2022; 131 (9):768-787 - 101.
Yang L, Liang H, Shen L, Guan Z, Meng X. LncRNA Tug1 involves in the pulmonary vascular remodeling in mice with hypoxic pulmonary hypertension via the microRNA-374c-mediated Foxc1. Life Sciences. 2019; 237 :116769 - 102.
Su H, Xu X, Yan C, et al. LncRNA H19 promotes the proliferation of pulmonary artery smooth muscle cells through AT1R via sponging let-7b in monocrotaline-induced pulmonary arterial hypertension. Respiratory Research. 2018; 19 (1):254 - 103.
Su H, Liu B, Chen H, et al. LncRNA ANRIL mediates endothelial dysfunction through BDNF downregulation in chronic kidney disease. Cell Death & Disease. 2022; 13 (7):661 - 104.
Zahid KR, Raza U, Chen J, Raj UJ, Gou D. Pathobiology of pulmonary artery hypertension: Role of long non-coding RNAs. Cardiovascular Research. 2020; 116 (12):1937-1947 - 105.
Gong Q , Hu Z, Jin Q , et al. Identification of JPX-RABEP1 pair as an immune-related biomarker and therapeutic target in pulmonary arterial hypertension by bioinformatics and experimental analyses. International Journal of Molecular Sciences. 2022; 23 (24) - 106.
Zhuo Y, Zeng Q , Zhang P, Li G, Xie Q , Cheng Y. Functional polymorphism of lncRNA MALAT1 contributes to pulmonary arterial hypertension susceptibility in Chinese people. Clinical Chemistry and Laboratory Medicine. 2017; 55 (1):38-46 - 107.
Wang X, Li Q , He S, et al. LncRNA FENDRR with m6A RNA methylation regulates hypoxia-induced pulmonary artery endothelial cell pyroptosis by mediating DRP1 DNA methylation. Molecular Medicine. 2022; 28 (1):126 - 108.
Song R, Lei S, Yang S, Wu SJ. LncRNA PAXIP1-AS1 fosters the pathogenesis of pulmonary arterial hypertension via ETS1/WIPF1/RhoA axis. Journal of Cellular and Molecular Medicine. 2021; 25 (15):7321-7334 - 109.
Han S, Chen X, Huang L. The tumor therapeutic potential of long non-coding RNA delivery and targeting. Acta Pharmaceutica Sinica B. 2023; 13 (4):1371-1382 - 110.
Peng Y, Huang X, Wang H. Serum lncRNA LINC01535 as biomarker of diagnosis, prognosis, and disease progression in breast cancer. Clinical Breast Cancer. 2023; 23 (6):620-627 - 111.
Hsu XR, Wu JE, Wu YY, et al. Exosomal long noncoding RNA MLETA1 promotes tumor progression and metastasis by regulating the miR-186-5p/EGFR and miR-497-5p/IGF1R axes in non-small cell lung cancer. Journal of Experimental & Clinical Cancer Research. 2023; 42 (1):283 - 112.
Bourdoumis A, Papatsoris AG, Chrisofos M, Efstathiou E, Skolarikos A, Deliveliotis C. The novel prostate cancer antigen 3 (PCA3) biomarker. International Brazilian Journal of Urology. 2010; 36 (6):665-668; discussion 669 - 113.
Greco S, Zaccagnini G, Perfetti A, et al. Long noncoding RNA dysregulation in ischemic heart failure. Journal of Translational Medicine. 2016; 14 (1):183 - 114.
Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006; 442 (7099):199-202 - 115.
Aravin A, Gaidatzis D, Pfeffer S, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006; 442 (7099):203-207 - 116.
Halbach R, Miesen P, Joosten J, et al. A satellite repeat-derived piRNA controls embryonic development of Aedes. Nature. 2020; 580 (7802):274-277 - 117.
Guo Z, Li Y, Ding SW. Small RNA-based antimicrobial immunity. Nature Reviews. Immunology. 2019; 19 (1):31-44 - 118.
Ma C, Zhang L, Wang X, et al. piRNA-63076 contributes to pulmonary arterial smooth muscle cell proliferation through acyl-CoA dehydrogenase. Journal of Cellular and Molecular Medicine. 2020; 24 (9):5260-5273 - 119.
Lipps C, Northe P, Figueiredo R, et al. Non-invasive approach for evaluation of pulmonary hypertension using extracellular vesicle-associated small non-coding RNA. Biomolecules. 2019; 9 (11):666 - 120.
Liu Y, Dou M, Song X, et al. The emerging role of the piRNA/piwi complex in cancer. Molecular Cancer. 2019; 18 (1):123 - 121.
Mai D, Ding P, Tan L, et al. PIWI-interacting RNA-54265 is oncogenic and a potential therapeutic target in colorectal adenocarcinoma. Theranostics. 2018; 8 (19):5213-5230 - 122.
Esteller M. Non-coding RNAs in human disease. Nature Reviews. Genetics. 2011; 12 (12):861-874 - 123.
Maxwell ES, Fournier MJ. The small nucleolar RNAs. Annual Review of Biochemistry. 1995; 64 :897-934 - 124.
Mallardo M, Poltronieri P, D'Urso OF. Non-protein coding RNA biomarkers and differential expression in cancers: A review. Journal of Experimental & Clinical Cancer Research. 2008; 27 (1):19 - 125.
Jaé N, Dimmeler S. Noncoding RNAs in vascular diseases. Circulation Research. 2020; 126 (9):1127-1145 - 126.
Al Tabosh T, Liu H, Koça D, et al. Impact of heterozygous ALK1 mutations on the transcriptomic response to BMP9 and BMP10 in endothelial cells from hereditary hemorrhagic telangiectasia and pulmonary arterial hypertension donors. Angiogenesis. 2024; 27 (2):211-227 - 127.
Chen Q , Yan M, Cao Z, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016; 351 (6271):397-400 - 128.
Waldron D. Non-coding RNA: Inheritance of diet-induced metabolic changes via tsRNAs. Nature Reviews. Genetics. 2016; 17 (3):128 - 129.
Park J, Ahn SH, Shin MG, Kim HK, Chang S. tRNA-derived small RNAs: Novel epigenetic regulators. Cancers (Basel). 2020; 12 (10):2773 - 130.
Chen Y, Tang Y, Hou S, et al. Differential expression spectrum and targeted gene prediction of tRNA-derived small RNAs in idiopathic pulmonary arterial hypertension. Frontiers in Molecular Biosciences. 2023; 10 :1204740 - 131.
Diener C, Keller A, Meese E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends in Genetics. 2022; 38 (6):613-626 - 132.
Finer M, Glorioso J. A brief account of viral vectors and their promise for gene therapy. Gene Therapy. 2017; 24 (1):1-2 - 133.
Savenkova DA, Makarova AA, Shalik IK, Yudkin DV. miRNA pathway alteration in response to non-coding RNA delivery in viral vector-based gene therapy. International Journal of Molecular Sciences. 2022; 23 (23):14954 - 134.
Samulski RJ, Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annual Review of Virology. 2014; 1 (1):427-451 - 135.
Saraiva J, Nobre RJ, Pereira de Almeida L. Gene therapy for the CNS using AAVs: The impact of systemic delivery by AAV9. Journal of Controlled Release. 2016; 241 :94-109 - 136.
Verdera HC, Kuranda K, Mingozzi F. AAV vector immunogenicity in humans: A long journey to successful gene transfer. Molecular Therapy. 2020; 28 (3):723-746 - 137.
Bulcha JT, Wang Y, Ma H, Tai P, Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduction and Targeted Therapy. 2021; 6 (1):53 - 138.
Ghebremedhin B. Human adenovirus: Viral pathogen with increasing importance. European Journal of Microbiology and Immunology. 2014; 4 (1):26-33 - 139.
Allen RJ, Byrnes AP. Interaction of adenovirus with antibodies, complement, and coagulation factors. FEBS Letters. 2019; 593 (24):3449-3460 - 140.
Lee CS, Bishop ES, Zhang R, et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes & Diseases. 2017; 4 (2):43-63 - 141.
Sweeney NP, Vink CA. The impact of lentiviral vector genome size and producer cell genomic to gag-pol mRNA ratios on packaging efficiency and titre. Molecular Therapy - Methods & Clinical Development. 2021; 21 :574-584 - 142.
Moraes FC, Pichon C, Letourneur D, Chaubet F. miRNA delivery by nanosystems: State of the art and perspectives. Pharmaceutics. 2021; 13 (11):1901 - 143.
Alameh MG, Tombácz I, Bettini E, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021; 54 (12):2877-2892.e7 - 144.
Chen Y, Li Z, Chen X, Zhang S. Long non-coding RNAs: From disease code to drug role. Acta Pharmaceutica Sinica B. 2021; 11 (2):340-354 - 145.
Witzigmann D, Kulkarni JA, Leung J, Chen S, Cullis PR, van der Meel R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Advanced Drug Delivery Reviews. 2020; 159 :344-363 - 146.
Springer AD, Dowdy SF. GalNAc-siRNA conjugates: Leading the way for delivery of RNAi therapeutics. Nucleic Acid Therapeutics. 2018; 28 (3):109-118 - 147.
Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Advanced Drug Delivery Reviews. 2014; 66 :110-116 - 148.
García-Pinel B, Porras-Alcalá C, Ortega-Rodríguez A, et al. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials (Basel). 2019; 9 (4):638 - 149.
Cano A, Ettcheto M, Chang JH, et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer's disease mice model. Journal of Controlled Release. 2019; 301 :62-75 - 150.
Cano A, Sánchez-López E, Ettcheto M, et al. Current advances in the development of novel polymeric nanoparticles for the treatment of neurodegenerative diseases. Nanomedicine (London, England). 2020; 15 (12):1239-1261 - 151.
Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. Journal of Controlled Release. 2001; 70 (1-2):1-20 - 152.
Owens DE 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International Journal of Pharmaceutics. 2006; 307 (1):93-102 - 153.
Tanno T, Zhang P, Bailey C, et al. A novel aptamer-based small RNA delivery platform and its application to cancer therapy. Genes & Diseases. 2023; 10 (3):1075-1089 - 154.
Rampado R, Caliceti P, Agostini M. Latest advances in biomimetic cell membrane-coated and membrane-derived Nanovectors for biomedical applications. Nanomaterials (Basel). 2022; 12 (9):1543 - 155.
Fang RH, Kroll AV, Gao W, Zhang L. Cell membrane coating nanotechnology. Advanced Materials. 2018; 30 (23):e1706759 - 156.
Cui J, Wang X, Li J, et al. Immune exosomes loading self-assembled nanomicelles traverse the blood-brain barrier for chemo-immunotherapy against glioblastoma. ACS Nano. 2023 [Online ahead of print] - 157.
Myo AC, Kobayashi Y, Niki Y, Kamimoto H, Moriyama K. Exosome-mediated small interfering RNA delivery inhibits aberrant osteoblast differentiation in apert syndrome model mice. Archives of Oral Biology. 2023; 153 :105753 - 158.
Arya SB, Collie SP, Parent CA. The ins-and-outs of exosome biogenesis, secretion, and internalization. Trends in Cell Biology. 2024; 34 (2):90-108 - 159.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020; 367 (6478):eaau6977 - 160.
Darband SG, Mirza-Aghazadeh-Attari M, Kaviani M, et al. Exosomes: Natural nanoparticles as bio shuttles for RNAi delivery. Journal of Controlled Release. 2018; 289 :158-170 - 161.
Li X, Yu Q , Zhao R, et al. Designer exosomes for targeted delivery of a novel therapeutic cargo to enhance sorafenib-mediated ferroptosis in hepatocellular carcinoma. Frontiers in Oncology. 2022; 12 :898156 - 162.
Zhong R, Talebian S, Mendes BB, et al. Hydrogels for RNA delivery. Nature Materials. 2023; 22 (7):818-831 - 163.
Segel M, Lash B, Song J, et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science. 2021; 373 (6557):882-889 - 164.
Gutkin A, Rosenblum D, Peer D. RNA delivery with a human virus-like particle. Nature Biotechnology. 2021; 39 (12):1514-1515 - 165.
Mohsen MO, Gomes AC, Vogel M, Bachmann MF. Interaction of viral capsid-derived virus-like particles (VLPs) with the innate immune system. Vaccines (Basel). 2018; 6 (3):37 - 166.
Morris GM, Lim-Wilby M. Molecular docking. Methods in Molecular Biology. 2008; 443 :365-382 - 167.
Ezkurdia I, Juan D, Rodriguez JM, et al. Multiple evidence strands suggest that there may be as few as 19, 000 human protein-coding genes. Human Molecular Genetics. 2014; 23 (22):5866-5878 - 168.
Zhou Y, Jiang Y, Chen SJ. RNA-ligand molecular docking: Advances and challenges. Wiley Interdisciplinary Reviews: Computational Molecular Science. 2022; 12 (3):e1571 - 169.
Totrov M, Abagyan R. Flexible ligand docking to multiple receptor conformations: A practical alternative. Current Opinion in Structural Biology. 2008; 18 (2):178-184 - 170.
Jiang Y, Chen SJ. RLDOCK method for predicting RNA-small molecule binding modes. Methods. 2022; 197 :97-105 - 171.
Sun LZ, Jiang Y, Zhou Y, Chen SJ. RLDOCK: A new method for predicting RNA-ligand interactions. Journal of Chemical Theory and Computation. 2020; 16 (11):7173-7183 - 172.
Gathiaka S, Liu S, Chiu M, et al. D3R grand challenge 2015: Evaluation of protein-ligand pose and affinity predictions. Journal of Computer-Aided Molecular Design. 2016; 30 (9):651-668 - 173.
Moult J, Pedersen JT, Judson R, Fidelis K. A large-scale experiment to assess protein structure prediction methods. Proteins. 1995; 23 (3):ii-v - 174.
Huang N, Shoichet BK, Irwin JJ. Benchmarking sets for molecular docking. Journal of Medicinal Chemistry. 2006; 49 (23):6789-6801 - 175.
Li Y, Han L, Liu Z, Wang R. Comparative assessment of scoring functions on an updated benchmark: 2. Evaluation methods and general results. Journal of Chemical Information and Modeling. 2014; 54 (6):1717-1736 - 176.
Li Y, Su M, Liu Z, et al. Assessing protein-ligand interaction scoring functions with the CASF-2013 benchmark. Nature Protocols. 2018; 13 (4):666-680 - 177.
Mysinger MM, Carchia M, Irwin JJ, Shoichet BK. Directory of useful decoys, enhanced (DUD-E): Better ligands and decoys for better benchmarking. Journal of Medicinal Chemistry. 2012; 55 (14):6582-6594 - 178.
Su M, Yang Q , Du Y, et al. Comparative assessment of scoring functions: The CASF-2016 update. Journal of Chemical Information and Modeling. 2019; 59 (2):895-913