Informations on the 60 most important species of Compositae weeds.
Abstract
Biological invasion is a global ecological problem, and it is important to understand the mechanism of successful invasion for the prevention and control of invasive weeds. Based on my experience and expertise in ecology, I have observed a significant gap in the literature regarding Compositae weeds invasions, and aimed to address this gap. We searched the literature related to Compositae weeds invasions published after 2000 in the China National Knowledge Infrastructure, PubMed, Scopus, Embase, and Web of Science. A list of 60 major Compositae weeds that are widely invasive around the world, and five important reasons (reproductive strategies, ecological adaptations, genetic diversity, enemy release, and human activities) explored that could be responsible for the powerful invasiveness of Compositae weeds. We offer a comprehensive overview of the current state of knowledge in this field and present a different perspective that incorporates existing theories. A clear address about the aggressive invasiveness of invasive species belonging to Compositae, and proposing scientific prevention, control, and management strategies will help prevent further invasion around the world in the future.
Keywords
- biological invasions
- Compositae weeds
- reproductive strategies
- ecological adaptations
- genetic diversity
- enemy release
- human activities
1. Introduction
Invasive weed species pose a significant threat to global ecosystems and economy around the world [1]. Compositae weeds have been particularly successful at invading heterogeneous habitats, many species within this family have become invasive in various regions (Table 1, contains references [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61]), especially in agroecological zones and grassland areas [62, 63]. Here, we aim to explore the factors that contribute to the strong invasiveness of Compositae weeds. Several reasons for their success in invading new habitats will be discussed, including their reproductive strategies, ecological adaptations, genetic diversity, enemy release, and the impacts of human activities on their spread (Figure 1). Understanding these factors can aid in the development of effective management strategies for controlling the invasion of Compositae weeds.
| Compositae weeds | Common name | Place origin | Invasive habitats | References |
|---|---|---|---|---|
| sticky snakeroot | Mexico | sparse vegetation, bare land | Poudel et al. [2] | |
| tropical whiteweed | Tropical America | valley, understory, meadow, wasteland | Erida et al. [3] | |
| annual ragweed | Central and North America | roadside, channels, riverbanks, streets | Gusev et al. [4] | |
| great ragweed | North America | fields, roadsides, wetlands | Xu et al. [5] | |
| annual saltmarsh aster | North America | roadside, abandoned land, wilderness | Xu et al. [6] | |
| common beggar’s tick | Tropical America | villageside, roadside, wasteland | Wang et al. [7] | |
| devil’s beggartick | North America | wet field | Min et al. [8] | |
| hairy beggarticks | America | villageside, roadside, wasteland | Li et al. [9] | |
| Siam weed | Mexico | hilly land, savanna | Xu et al. [10] | |
| annual fleabane | North America | hillsides, roadsides, fields | Huang et al. [11] | |
| horseweed | North America | wilderness, wasteland, field edge, roadside | Liendo et al. [12] | |
| fleabane daisy | South America | meadow, wilderness, roadside | Maslo et al. [13] | |
| bristly yellowtop | South America | wilderness, pasture, abandoned farmland | Dai et al. [14] | |
| American rope | Central and South America | forest, farmland | Jiang et al. [15] | |
| famine weed | Tropical America | open land, roadside, riverside, slopes | Ullah et al. [16] | |
| fleabane | South America | roadside, wasteland, farmland, grassland | Intanon et al. [17] | |
| Canada goldenrod | North America | river beach, wasteland, roadside, farmland side | Tian et al. [18] | |
| tree marigold | Mexico | river beach, roadside, farmland | Jiao et al. [19] | |
| flossflower | Tropical America | forest edge, riverside, farmland, grassland | El Hadidy et al. [20] | |
| redflower ragleaf | Africa | underwood, bushes, beside ditches | Xie et al. [21] | |
| Brazilian fleabane | South America | roadside, river embankment, hillside, countryside | Qasem et al. [22] | |
| gallant soldier | South America | roadside, open space | Ripanda et al. [23] | |
| gallant soldier | Mexico | forest, roadside | Liu et al. [24] | |
| wedelia | Tropical America | seaside, waterside, limestone areas | Zhang et al. [25] | |
| Italian cocklebur | Europe, North America | wasteland, waterside, farmland | Shi et al. [26] | |
| spiny cocklebur | America | roadside, wasteland, farmland | Dudás et al. [27] | |
| Spanish needles | America | wastelands, hillsides, fields | Zhuang et al. [28] | |
| lanceleaf coreopsis | USA | woods, mountains | Kim et al. [29] | |
| sulfur cosmos | Mexico | pastoral, sandy land | Liu et al. [30] | |
| giant false ragweed | North America | highway, the manure pile | Abramova & Nurmieva [31] | |
| Philadelphia fleabane | North America | roadside, wilderness, hillside, orchard, forest | Xu et al. [32] | |
| wild marigold | Tropical America | alpine areas | Moghaddam et al. [33] | |
| French marigold | Mexico | grassland, forest, garden | Prebeg et al. [34] | |
| western salsify | Central Asia, Europe | river beach, wasteland, field edge | Jordon-Thaden et al. [35] | |
| Mongolian cocklebur | Mexico | roadside, ditchside, field edge, grassland | Han et al. [36] | |
| chicory | Europe, West Central Asia, North Africa | wasteland, prairie, field, slope | Gazwi et al. [37] | |
| false daisy | America | riverside, fieldside, roadside | Timalsina & Devkota [38] | |
| pilewort | Tropical America | understory, hillsides, shrubs, wetlands | Hung et al. [39] | |
| American burnweed | Tropical America | fieldside, roadside | Funez et al. [40] | |
| corn marigold | Morocco | pastoral, wasteland | Mircea et al. [41] | |
| Jerusalem artichoke | North America | ruins, houseside, roadside | Phongphan et al. [42] | |
| common groundsel | Europe | grassland, hillside, roadside | Ebadi & Eftekharian [43] | |
| milk thistle | West Asia, North Africa, Southern Europe | open space, wasteland, roadside | Hossain et al. [44] | |
| annual sowthistle | South America | wasteland, field | Ghoshal et al. [45] | |
| prickly sowthistle | Europe, the Mediterranean | hillside, forest edge, waterside | Sidhu et al. [46] | |
| common sowthistle | Europe,d the Mediterranean | forest, field, open space | Choudhary et al. [47] | |
| dandelion | Europe | grassland, forest, field, roadside | Watanabe et al. [48] | |
| Peruvian zinnia | Mexico | hillside, grass, roadside | Mohamed et al. [49] | |
| bristly starbur | South America | flat slopes, riversides, ditchsides, roadsides | Sukholozova et al. [50] | |
| paracress | South America | fieldside, roadside | Kato-Noguchi et al. [51] | |
| corn mayweed | Europe | roadside | Wozniak et al. [52] | |
| yellow chamomile | Europe | parks, fields | Orlando et al. [53] | |
| Cuban aster | Caribbean | seaside, wetland | Cheng et al. [54] | |
| straggler daisy | Cuba, Mexico and the United States | wilderness, cultivated land, roadside, houseside | Lal et al. [55] | |
| cornflower | Europe | wasteland, field | Palma-Bautista et al. [56] | |
| diffuse knapweed | West Asia, Europe | wasteland, field | Keever et al. [57] | |
| spotted knapweed | Europe | wasteland, field | Mummey et al. [58] | |
| goldenmane tickseed | North America | parks, gardens | Crawford & Smith [59] | |
| large-flowered tickseed | USA | wasteland, mountains | Huang et al. [60] | |
| golden tickseed | USA | wasteland, mountains, field | Jiang et al. [61] |
Table 1.
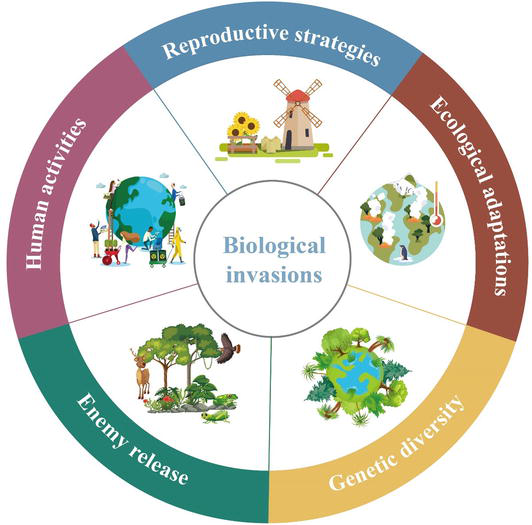
Figure 1.
Main reasons for the global invasion success of Compositae weeds. Five important reasons can be responsible for explaining the strong invasiveness of Compositae weeds, including reproductive strategies, ecological adaptations, genetic diversity, enemy release, and human activities.
Compositae weeds are recognized for their invasive tendencies, presenting significant challenges such as decreased biodiversity and habitat degradation [64]. These invasive plants possess traits like rapid growth, prolific seed production, and adaptability to various environmental conditions, which enable them to outcompete native vegetation and dominate ecosystems [65]. The spread of invasive Compositae weeds is facilitated by human activities, habitat disturbances, and the absence of natural predators in new habitats [66]. Effective control measures, including mechanical removal and targeted herbicide application, are essential to manage their invasion and safeguard native ecosystems from further disruption [67, 68].
Compositae weeds display a wide range of life forms, including annuals, biennials, and perennials [69]. They are characterized by their composite flower heads, which consist of multiple individual flowers on a single head [70]. Compositae weeds have become widely distributed around the world due to their excellent adaptability to different environments and their high reproduction rates [71]. As a result, many species within this family have been introduced to new areas, where they often outcompete and displace native species [72]. For example, Italian cocklebur (Figure 2). Understanding the reasons behind their invasiveness is crucial for effective management and conservation practices.

Figure 2.
The current invasion status of Compositae plant
2. Methodology
The China National Knowledge Infrastructure, PubMed, Scopus, Embase, and Web of Science search engines were used in the literature collection [73]. Only journal articles and reviews that were published English after 2000 in this study [74]. The search terms and strategies are as follows: TS = (“biological invasions*”) OR TI = (“Compositae/Asteraceae weeds*”) OR TI = (“strong invasiveness*”) OR TI = (“successful invasion*”) [75].
3. Reasons for the strong invasiveness of Compositae weeds
3.1 Reproductive strategies
One of the key factors contributing to the invasiveness of Compositae weeds is their unique reproductive strategies. Many species within this family produce large quantities of small, lightweight seeds that are easily dispersed over long distances by wind or water [76]. Additionally, their ability to asexual reproduction, self-pollinate, and insect pollination allows them to rapidly colonize new habitats [77]. These reproductive characteristics provide Compositae weeds with a competitive advantage, allowing them to establish and dominate over other native weed species. Such as
3.2 Ecological adaptations
Compositae weeds exhibit various ecological adaptations that contribute to their invasiveness. They are known for their ability to thrive in disturbed habitats, such as roadsides, fields, and forests. Their wide tolerance to different soil types, pH levels, and moisture conditions also enables them to occupy diverse ecological niches [78]. Furthermore, Compositae weeds often possess allelopathic compounds that inhibit the growth of neighboring weeds, further enhancing their ability to outcompete native species [79]. Such as
3.3 Genetic diversity
Genetic diversity plays a crucial role in the invasiveness of Compositae weeds. Species within this family often have high genetic variability, which allows them to adapt to new environments and overcome biotic and abiotic stresses [80]. This genetic diversity also increases the chances of hybridization and the formation of novel genotypes with increased invasiveness. Additionally, the presence of polyploid species within Compositae contributes to their ability to occupy new habitats and rapidly expand their range, such as
3.4 Enemy release
In their native range, Compositae weeds coexist with specialized herbivores, diseases, and pathogens, regulating their population growth [81]. However, when introduced to new geographic regions, they often escape from their natural enemies, enabling population growth without significant constraints. This lack of natural enemies can lead to uncontrolled proliferation and invasion of Compositae weeds, posing a threat to native biodiversity, such as
3.5 Human activities
Human activities such as agriculture, horticulture, and international trade have significantly facilitated the spread of Compositae weeds [82]. For example, their introduction as ornamental weeds has resulted in accidental escapes and subsequent invasions in many parts of the world. Furthermore, the disturbance of natural ecosystems through land clearing, urbanization, and climate change creates favorable conditions for the establishment and spread of invasive Compositae species [83]. Prevention measures to control their introduction and spread should be implemented to minimize their impact on native biodiversity, such as
4. Future management strategies of Compositae plant invasions
Effective management strategies for controlling invasive weeds typically involve a combination of prevention, early detection, eradication, and ongoing monitoring. Prevention efforts include implementing strict regulations on the importation and sale of potentially invasive Compositae species, as well as raising public awareness about the risks associated with introducing non-native Compositae plants into natural ecosystems [84].
Early detection is crucial for addressing invasive Compositae weeds before they become established and widespread. This involves training volunteers and professionals to identify invasive Compositae plants and implementing surveillance programs to quickly detect and respond to new invasions [85].
Eradication methods vary depending on the invasive Compositae species and the extent of the invasion but may include mechanical methods such as hand-pulling, mowing, or cutting, as well as chemical control methods like herbicide application. Biological control, using natural enemies such as insects or pathogens to suppress invasive Compositae weeds, can also be an effective long-term strategy when implemented carefully to minimize unintended consequences [86, 87, 88, 89].
Ongoing monitoring and management are essential to prevent the re-establishment and spread of invasive Compositae plants. This includes regular surveys to detect and treat new invasions, as well as restoration efforts to rehabilitate areas impacted by invasive weeds and promote the recovery of native plant communities [90].
Collaboration among government agencies, land managers, researchers, and the public is critical for successful invasive Compositae weeds management. By implementing integrated and adaptive management approaches, we can work toward reducing the impact of invasive Compositae weeds and preserving the health and biodiversity of our local ecosystems, especially in agricultural production areas [91].
5. Conclusions
Here, we present a list of the 60 most important Compositae invasive weeds around the world and discuss the reasons why they are so invasive. The aggressive invasiveness of Compositae weeds can be attributed to a combination of factors such as their reproductive strategies, ecological adaptations, genetic diversity, enemy release, and the influence of human activities. Understanding the mechanisms driving their invasiveness is essential for managing and controlling the spread of these species. Further research is needed to assess the impacts of different control measures and develop effective strategies to prevent the further spread of invasive Compositae weeds and protect native ecosystems.
Acknowledgments
This work is supported by the Central Public-Interest Scientific Institution Basal Research Fund of China (fund No. 1610012024003).
Author contribution statement
H. Yang and J.S. Tang collected the data and H. Yang wrote the manuscript, and J.S. Tang revised the manuscript.
References
- 1.
Diagne C, Leroy B, Vaissière AC, et al. High and rising economic costs of biological invasions worldwide. Nature. 2021; 592 :571-576. DOI: 10.1038/s41586-021-03405-6 - 2.
Poudel AS, Jha PK, Shrestha BB, et al. Biology and management of the invasive weed Ageratina adenophora (Asteraceae): Current state of knowledge and future research needs. Weed Research. 2019;59 :79-92. DOI: 10.1111/wre.12351 - 3.
Erida G, Ichsan CN, Syamsuddin, et al. Potential of secondary metabolites of Ageratum conyzoides L. in weed management: A review. Allelopathy Journal. 2023;58 :23-40. DOI: 10.26651/allelo.j/2023-58-1-1417 - 4.
Gusev AP. Invasion of ambrosia artemisifolia L. into the landscapes of the southeastern Belarus. Russian Journal of Biological Invasions. 2019;10 :129-135. DOI: 10.1134/S2075111719020061 - 5.
Xu K, Liu XY, Zhao CX, et al. Nitrogen deposition further increases Ambrosia trifida root exudate invasiveness under global warming. Environmental Monitoring and Assessment. 2023;195 :759. DOI: 10.1007/s10661-023-11380-w - 6.
Xu ZL, Zhong SS, Yu YL, et al. Drought stress intensifies the phytotoxicity of five Asteraceae exotic invasive plants. Israel Journal of Plant Sciences. 2023; 70 :162-172. DOI: 10.1163/22238980-bja10078 - 7.
Wang Y, Lian JY, Shen H, et al. The effects of Bidens alba invasion on soil bacterial communities across different coastal ecosystem land-use types in southern China. PLoS One. 2021;16 :e0253358. DOI: 10.1371/journal.pone.0253358 - 8.
Min GG, Park TS, Park JS, et al. First report of cucumber mosaic virus infecting Bidens frondosa (devil's beggarticks) in Korea. Journal of Plant Pathology. 2023;105 :1739-1740. DOI: 10.1007/s42161-023-01493-z - 9.
Li YN, Gu YS, Li MZ, et al. Comparison on the phytoextraction efficiency of Bidens pilosa at heavy metal contaminated site in natural and electrokinetic conditions. Journal of Groundwater Science and Engineering. 2021;9 :121-128. DOI: 10.19637/j.cnki.2305-7068.2021.02.004 - 10.
Xu QY, Wang D, Quan GM, et al. Pennisetum Hydridum 's potential for controlling invasiveChromolaena Odorata . Sustainability. 2019;11 :5990. DOI: 10.3390/su11215990 - 11.
Huang YM, Zhang GL, Fu WD, et al. Impacts of climate change on climatically suitable regions of two invasive erigeron weeds in China. Frontiers in Plant Science. 2023;14 :1238656. DOI: 10.3389/fpls.2023.1238656 - 12.
Liendo D, García-Mijangos I, Biurrun I, et al. Annual weedy species of erigeron in the northern Iberian Peninsula: A review. Mediterranean Botany. 2021;42 :e67649. DOI: 10.5209/mbot.67649 - 13.
Maslo S, Saric S. Erigeron sumatrensis Retz. (Compositae), a recently recognized invasive alien species in Bosnia and Herzegovina. Glasnik Hrvatskog Botanickog Drustva. 2020;8 :88-93. DOI: 10.46232/glashbod.8.2.3 - 14.
Dai L, Wu LL, Zhou XR, et al. Effects of water extracts of Flaveria bidentis on the seed germination and seedling growth of three plants. Scientific Reports. 2022;12 :17700. DOI: 10.1038/s41598-022-22527-z - 15.
Jiang ZY, Wang YT, Zheng YP, et al. Physiological and transcriptomic responses of Mikania micrantha stem to shading yield novel insights into its invasiveness. Biological Invasions. 2021;23 :2927-2943. DOI: 10.1007/s10530-021-02546-z - 16.
Ullah S, Shakir M, Iqbal MS, et al. Identifying optimal waveband positions for discriminating Parthenium hysterophorus using hyperspectral data. Ecological Informatics. 2021;64 :101362. DOI: 10.1016/j.ecoinf.2021.101362 - 17.
Intanon S, Wiengmoon B, Mallory-Smith CA. Seed morphology and allelopathy of invasive Praxelis clematidea . Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2020;48 :261-272. DOI: 10.15835/nbha48111831 - 18.
Tian ZS, Cheng JL, Xu JX. Cytogeography of naturalized Solidago canadensis populations in Europe. Plants-Basel. 2023;12 :1113. DOI: 10.3390/plants12051113 - 19.
Jiao Y, Cheng XP, Wang SH, et al. Characteristics of allometric growth of Tithonia diversifolia , an alien invasive plant. Journal of West China Forestry Science. 2020;49 :156-161. DOI: 10.16473/j.cnki.xblykx1972.2020.01.024 - 20.
El Hadidy D, El Sayed AM, El Tantawy M, et al. Larvicidal and repellent potential of Ageratum houstonianum againstCulex pipiens . Scientific Reports. 2022;12 :21410. DOI: 10.1038/s41598-022-25939-z - 21.
Xie YD, Wang LM, Yang LX, et al. Intercropping with Eclipta prostrata andCrassocephalum crepidioides decrease cadmium uptake of tomato seedlings. International Journal of Environmental Analytical Chemistry. 2021;101 :1231-1239. DOI: 10.1080/03067319.2019.1678606 - 22.
Qasem JR. Chemical control and herbicide resistance of hairy fleabane ( Erigeron bonariensis L.) in Jordan. PLoS One. 2023;18 :e0263154. DOI: 10.1371/journal.pone.0263154 - 23.
Ripanda A, Luanda A, Sule KS, et al. Galinsoga parviflora (Cav.): A comprehensive review on ethnomedicinal, phytochemical and pharmacological studies. Heliyon. 2023;9 :e13517. DOI: 10.1016/j.heliyon.2023.e13517 - 24.
Liu RL, Zhang WG, Lee BR, et al. Rhizosphere and root fungal community of the invasive plant Galinsoga quadriradiata changes along its elevational expansion route. Journal of Plant Ecology. 2023;16 :rtac055. DOI: 10.1093/jpe/rtac055 - 25.
Zhang QL, Chen GX, Shao L, et al. The hybridization between Sphagneticola trilobata (L.) Pruski andSphagneticola calendulacea (L.) Pruski improved the tolerance of hybrid to cadmium stress. Chemosphere. 2020;249 :126540. DOI: 10.1016/j.chemosphere.2020.126540 - 26.
Shi T, Long ZZ, Miao M. Glomus mosseae promotesXanthium italicum invasion. Sains Malaysiana. 2020;49 :2425-2432. DOI: 10.17576/jsm-2020-4910-08 - 27.
Dudás M, Eliás P. Alien weed Xanthium spinosum in Slovakia I: Distribution and habitats. Journal of Central European Agriculture. 2021;22 :305-316. DOI: 10.5513/JCEA01/22.2.3083 - 28.
Zhuang G, Wang YQ , Li SJ, et al. Tissue distribution and molecular docking research on the active components of Bidens bipinnata L. against hyperlipidemia. Biomedical Chromatography. 2021;35 :e5026. DOI: 10.1002/bmc.5026 - 29.
Kim HG, Oh HJ, Ko JH, et al. New flavonoids from the flowers of Coreopsis lanceolata and their pharmacological activities. Planta Medica. 2019;85 :1495-1496. DOI: 10.1055/s-0039-3399913 - 30.
Liu XY, Ou H, Gregersen H, et al. Supercritical carbon dioxide extraction of Cosmos sulphureus seed oil with ultrasound assistance. Journal of CO2 Utilization. 2023;70 :102429. DOI: 10.1016/j.jcou.2023.102429 - 31.
Abramova LM, Nurmieva SV. On the ecology and biology of invasive species Cyclachaena xanthiifolia (Nutt.) Fresen. in the southern Urals and Cisural region. Russian Journal of Ecology. 2014;45 :249-255. DOI: 10.1134/s106741361404002x - 32.
Xu SS, Zhao YH, Yan J, et al. Light availability and anthropogenic stress shape plant understory invasions in understory of urban forests: A case study in Shanghai. Biological Invasions. 2023; 25 :3223-3236. DOI: 10.1007/s10530-023-03104-5 - 33.
Moghaddam M, Farhadi N, Panjtandoust M, et al. Seed germination, antioxidant enzymes activity and proline content in medicinal plant Tagetes minuta under salinity stress. Plant Biosystems. 2020;154 :835-842. DOI: 10.1080/11263504.2019.1701122 - 34.
Prebeg T, Bedran S, Zutic I. The effect of mechanical stress on transplants of three ornamental Asteraceae species. Journal of Central European Agriculture. 2019; 20 :365-375. DOI: 10.5513/JCEA01/20.1.2063 - 35.
Jordon-Thaden IE, Spoelhof JP, Viccini LF, et al. Phenotypic trait variation in the north American Tragopogon allopolyploid complex. American Journal of Botany. 2023;110 :1-16. DOI: 10.1002/ajb2.16189 - 36.
Han J, Wang JW, Wang YC, et al. Sesquiterpene lactones-enriched fractions from Xanthium mongolicum Kitag alleviate RA by regulating M1 macrophage polarization via NF-κB and MAPK signaling pathway. Frontiers in Pharmacology. 2023;14 :1104153. DOI: 10.3389/fphar.2023.1104153 - 37.
Gazwi HSS, Mahmoud ME, Toson EMA. Analysis of the phytochemicals of Coriandrum sativum andCichorium intybus aqueous extracts and their biological effects on broiler chickens. Scientific Reports. 2022;12 :9964. DOI: 10.1038/s41598-022-14645-5 - 38.
Timalsina D, Devkota HP. Eclipta prostrata (L.) L. (Asteraceae): Ethnomedicinal uses, chemical constituents, and biological activities. Biomolecules. 2021;11 :1738. DOI: 10.3390/biom11111738 - 39.
Hung NH, Satyal P, Hieu HV, et al. Mosquito larvicidal activity of the essential oils of Erechtites species growing wild in Vietnam. Insects. 2019;10 :47. DOI: 10.3390/insects10020047 - 40.
Funez L, Hassemer G, Peroni N, et al. Taxonomic notes on Erechtites (Asteraceae: Senecioneae). Phytotaxa. 2021;489 :155-170. DOI: 10.11646/phytotaxa.489.2.4 - 41.
Mircea DM, Calone R, Shakya R, et al. Use of multivariate analysis in screening for drought tolerance in ornamental Asteraceae species. Agronomy-Basel. 2023; 13 :687. DOI: 10.3390/agronomy13030687 - 42.
Phongphan J, Wiyada M, Thanaset S, et al. Bioactive compounds from organic extracts of Helianthus tuberosus L. flowers. Industrial Crops and Products. 2018;119 :57-63. DOI: 10.1016/j.indcrop.2018.03.060 - 43.
Ebadi M, Eftekharian R. Morphological and genetic diversity of Senecio vulgaris L. (Asteraceae) in Iran. Acta Botanica Croatica. 2021;80 :125-130. DOI: 10.37427/botcro-2021-012 - 44.
Hossain MM, Cho SB, Kim IH. Silybum marianum seed extract as a potential phytogenic feed additive for improving growth performance and nutrient digestibility in growing pigs. Canadian Journal of Animal Science. 2023;00 :1-6. DOI: 10.1139/cjas-2023-0053 - 45.
Ghoshal PP, Padal SB, Anand K, et al. Soliva (Asteraceae: Anthemideae) - a new generic record to the flora of erstwhile Bihar (Jharkhand) with a note on its nomenclature. Indian Journal of Forestry. 2019;42 :177-180. DOI: 10.54207/bsmps1000-2019-4JWS8A - 46.
Sidhu MC, Rai S, Singh R. A cytomorphological investigation of three species of the genus Sonchus L. (Asterales: Asteraceae) from Punjab, India. Journal of Threatened Taxa. 2021;13 :19640-19644. DOI: 10.11609/jott.7367.13.11.19640-19644%20 - 47.
Choudhary VK, Dubey RP, Singh PK. Management of field sowthistle ( Sonchus oleraceus L.): An emerging threat in winter crops. Indian Journal of Weed Science. 2021;53 :142-145. DOI: 10.5958/0974-8164.2021.00026.5 - 48.
Watanabe K, Shibaike H, Suzuki T, et al. DNA contents and karyotypes of the natural hybrids in Taraxacum (Asteraceae) in Japan. Acta Phytotaxonomica et Geobotanica. 2021;72 :135-144. DOI: 10.18942/apg.202013 - 49.
Mohamed AM, Cifuente DA, Satorres SE, et al. Biological activity of roots and aerial parts of Zinnia peruviana on pathogenic micro-organisms in planktonic state and biofilm forming. Letters in Applied Microbiology. 2022;74 :419-428. DOI: 10.1111/lam.13622 - 50.
Sukholozova EA, Orlova JV, Kulakova YY, et al. Monitoring of the phytosanitary status of the hispid starburr in Primorsky Krai. Russian Journal of Biological Invasions. 2023; 14 :240-250. DOI: 10.1134/S2075111723020133 - 51.
Kato-Noguchi H, Suwitchayanon P, Boonmee S, et al. Plant growth inhibitory activity of the extracts of Acmella oleracea and its growth inhibitory substances. Natural Product Communications. 2019;14 :1-5. DOI: 10.1177/1934578X19858-805 - 52.
Wozniak A. Effect of cropping systems on quantitative changes in prevailing weed species. Agronomy Science. 2023; 78 :121-133. DOI: 10.24326/as.2023.5025 - 53.
Orlando G, Zengin G, Ferrante C, et al. Chemical profiles and pharmacological properties of two Anthemis species:Anthemis tinctoria var.pallida andA. Cretica subsp.tenuiloba . Planta Medica. 2019;85 :1416-1417. DOI: 10.1055/s-0039-3399708 - 54.
Cheng HY, Wang S, Wei M, et al. Effect of leaf water extracts of four Asteraceae alien invasive plants on germination performance of Lactuca sativa L. under acid deposition. Plant Ecology. 2021;222 :433-443. DOI: 10.1007/s11258-021-01117-5 - 55.
Lal R, Kaur A, Kaur S, et al. Nature of phytotoxic interference of alien weed ' Calyptocarpus vialis ' against some crop plants. Environmental Monitoring and Assessment. 2021;193 :334. DOI: 10.1007/s10661-021-09092-0 - 56.
Palma-Bautista C, Vázquez- García JG, de Portugal J, et al. Enhanced detoxification via Cyt-P450 governs cross-tolerance to ALS-inhibiting herbicides in weed species of Centaurea . Environmental Pollution. 2023;322 :121140. DOI: 10.1016/j.envpol.2023.121140 - 57.
Keever CC, Gültekin L, Bourchier RS, et al. Post-release genetic assessment of two congeneric weed biological control agents. Biological Control. 2021; 152 :104462. DOI: 10.1016/j.biocontrol.2020.104462 - 58.
Mummey DL, Rillig MC. The invasive plant species Centaurea maculosa alters arbuscular mycorrhizal fungal communities in the field. Plant and Soil. 2006;288 :81-90. DOI: 10.1007/s11104-006-9091-6 - 59.
Crawford DJ, Smith EB. Leaf flavonoid chemistry and taxonomy of coreopsis sect.Coreopsis . Biochemical Systematics and Ecology. 1985;13 :115-118. DOI: 10.1016/0305-1978(85)90068-7 - 60.
Huang YQ , Li BQ , Chen HF, et al. Gamma-aminobutyric acid enhances cadmium phytoextraction by Coreopsis grandiflora by remodeling the rhizospheric environment. Plants-Basel. 2023;12 :1484. DOI: 10.3390/plants12071484 - 61.
Jiang H, Li ZY, Jiang XM, et al. Physiological changes and transcript identification in Coreopsis tinctoria Nutt. in early stages of salt stress. Peer J. 2021;9 :e11888. DOI: 10.7717/peerj.11888 - 62.
Bieker VC, Battlay P, Petersen B, et al. Uncovering the genomic basis of an extraordinary weed invasion. Science Advances. 2022; 8 :eabo5115. DOI: 10.1126/sciadv.abo5115 - 63.
Tian BL, Pei YC, Huang W, et al. Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive weed. ISME Journal. 2021; 15 :1919-1930. DOI: 10.1038/s41396-021-00894-1 - 64.
Mcgaughran A, Dhami MK, Parvizi E, et al. Genomic tools in biological invasions: Current state and future frontiers. Genome Biology and Evolution. 2024; 16 :evad230. DOI: 10.1093/gbe/evad230 - 65.
Zhang Q , Wang YP, Liu X. Risk of introduction and establishment of alien vertebrate species in transboundary neighboring areas. Nature Communications. 2024; 15 :870. DOI: 10.1038/s41467-024-45025-4 - 66.
Croft L, Matheson P, Flemming C. Population structure and interspecific hybridisation of two invasive blowflies (Diptera: Calliphoridae ) following replicated incursions into New Zealand. Ecology and Evolution. 2024;14 :e10832. DOI: 10.1002/ece3.10832 - 67.
Chen YY, Gao YC, Huang XN. Incorporating adaptive genomic variation into predictive models for invasion risk assessment. Environmental Science and Ecotechnology. 2024; 18 :100299. DOI: 10.1016/j.ese.2023.100299 - 68.
Hulme PE, Ahmed DA, Haubrock PJ, et al. Widespread imprecision in estimates of the economic costs of invasive alien species worldwide. Science of the Total Environment. 2024; 909 :167997. DOI: 10.1016/j.scitotenv.2023.167997 - 69.
Mohammed HA, Qureshi KA, Ali HM, et al. Bio-evaluation of the wound healing activity of Artemisia Judaica L. as part of the weed’s use in traditional medicine; phytochemical, antioxidant, anti-inflammatory, and antibiofilm properties of the weed’s essential oils. Antioxidants (Basel). 2022;11 :332. DOI: 10.3390/antiox11020332 - 70.
Prusinkiewicz P, Zhang T, Owens A, et al. Phyllotaxis without symmetry: What can we learn from flower heads? Journal of Experimental Botany. 2022; 73 :3319-3329. DOI: 10.1093/jxb/erac101 - 71.
Liu B, Yan J, Li WH, et al. Mikania micrantha genome provides insights into the molecular mechanism of rapid growth. Nature Communications. 2020;11 :340. DOI: 10.1038/s41467-019-13926-4 - 72.
Hannula SE, Heinen R, Huberty M, et al. Persistence of plant-mediated microbial soil legacy effects in soil and inside roots. Nature Communications. 2021; 12 :5686. DOI: 10.1038/s41467-021-25971-z - 73.
Alemu SM, Tura AK, do Amaral GSG, et al. How applicable is geospatial analysis in maternal and neonatal health in sub-Saharan Africa? A systematic review, Journal of Globalization and Health 2022; 12 :04066. DOI: 10.7189/jogh.12.04066 - 74.
Zhao X. Stakeholder-associated factors influencing construction and demolition waste management: A systematic review. Buildings. 2021; 11 (4):149-149. DOI: 10.3390/buildings11040149 - 75.
Li M, Lu Y, Xu X. Mapping the scientific structure and evolution of renewable energy for sustainable development. Environmental Science and Pollution Research International. 2022; 29 (43):64832-64845. DOI: 10.1007/s11356-022-20361-4 - 76.
Eppinga MB, Baudena M, Haber EA, et al. Spatially explicit removal strategies increase the efficiency of invasive plant species control. Ecological Applications. 2021; 31 :e02257. DOI: 10.1002/eap.2257 - 77.
Giavi S, Fontaine C, Knop E. Impact of artificial light at night on diurnal plant-pollinator interactions. Nature Communications. 2021; 12 :1690. DOI: 10.1038/s41467-021-22011-8 - 78.
Zhang Y, Tang JS, Ren G, et al. Global potential distribution prediction of Xanthium italicum based on Maxent model. Scientific Reports. 2021;11 :16545. DOI: 10.1038/s41598-021-96041-z - 79.
Takemori A, Naiki A, Takakura KI, et al. Comparison of mechanisms of reproductive interference in Taraxacum . Annals of Botany. 2019;123 :1017-1027. DOI: 10.1093/aob/mcz007 - 80.
Tang JS, Mao KS, Zhang HY, et al. Multiple introductions and genetic admixture facilitate the successful invasion of Plantago virginica into China. Biological Invasions. 2022;24 :2261-2272. DOI: 10.1007/s10530-022-02773-y - 81.
Lin TT, Vrieling K, Laplanche D, et al. Evolutionary changes in an invasive plant support the defensive role of plant volatiles. Current Biology. 2021; 31 :3450-3456. DOI: 10.1016/j.cub.2021.05.055 - 82.
Ryan SF, Lombaert E, Espeset A, et al. Global invasion history of the agricultural pest butterfly Pieris rapae revealed with genomics and citizen science. Proceedings of the National Academy of Sciences of the United States of America. 2019;116 :2261-2272. DOI: 10.1073/pnas.1907492116 - 83.
Wang XX, Xiao XM, He Q , et al. Biological invasions in China’s coastal zone. Science. 2022; 378 :957. DOI: 10.1126/science.ade9665 - 84.
Botella C, Bonnet P, Hui C, et al. Dynamic species distribution modeling reveals the pivotal role of human-mediated long-distance dispersal in plant invasion. Biology (Basel). 2022; 11 (9):1293. DOI: 10.3390/biology11091293 - 85.
du Plessis NS, Rebelo AJ, Richardson DM, et al. Guiding restoration of riparian ecosystems degraded by plant invasions: Insights from a complex social-ecological system in the global south. Ambio. 2021; 51 :1552-1568. DOI: 10.1007/s13280-021-01691-y - 86.
Zhang L, Rohr J, Cui R, et al. Biological invasions facilitate zoonotic disease emergences. Nature Communications. 2022; 13 (1):1762. DOI: 10.1038/s41467-022-29378-2 - 87.
Tang JS, Liu Y, Zhang C, et al. Identification of pathogenic fungi causing leaf spot of Urtica cannabina andMalus sieversii in the wild fruit forest of Tianshan Mountain, Xinjiang, China. Sains Malaysiana. 2022;51 (7):2025-2032. DOI: 10.17576/jsm-2022-5107-07 - 88.
Tang JS, Huang L, Liu Y, et al. Two phytotoxins isolated from the pathogenic fungus of the invasive weed Xanthium italicum . Chemistry & Biodiversity. 2020;17 (4):e200004. DOI: 10.1002/cbdv.202000043 - 89.
Tang JS, Jiang CY, Liu Y, et al. Allelopathic potential of volatile organic compounds released by Xanthium sibiricum Patrin ex Widder. Allelopathy Journal. 2019;47 (2):233-241. DOI: 10.26651/allelo.j/2019-47-2-1234 - 90.
Wong MKL, Lee RH, Leong CM, et al. Trait-mediated competition drives an ant invasion and alters functional diversity. Proceedings of the Royal Society B-Biological Sciences. 1977; 2022 (289):20220504. DOI: 10.1098/rspb.2022.0504 - 91.
Lu M, Bond WJ, Sheffer E, et al. Biome boundary maintained by intense belowground resource competition in world's thinnest-rooted plant community. Proceedings of the National Academy of Sciences of the United States of America. 2022; 119 (9):e2117514119. DOI: 10.1073/pnas.2117514119