Abstract
The study of quantum thermodynamics has led to the development of quantum batteries. These devices use quantum advantages to store and extract useful energy from physical systems. Ergotropy is the maximum work that can be extracted from a quantum system by cyclic unitary operations. When external thermal baths couple with the quantum battery, there is energy loss due to thermal effects on the system. In some cases, a part of the total energy available in the system cannot be stored as ergotropy. Therefore, it is important to consider the amount of residual energy that cannot be extracted as useful work from quantum batteries by unitary processes. To better understand the amount of energy lost during work extraction, it is necessary to examine the constraint of unitary processes. The system exergy represents the maximum amount of work that can be extracted from the system while bringing it into equilibrium with a thermal bath. It can be separated into two parts: ergotropy and residual energy. Thus, the present chapter describes the relationship between exergy and its potential benefits and effects on the performance of quantum batteries.
Keywords
- quantum batteries
- ergotropy
- unitary process
- exergy
- quantum thermodynamics
1. Introduction
From the late eighteenth century until today, scientists have been experimenting with electrochemical reactions, which allow us to convert chemical energy into electricity. Despite centuries of innovation, the fundamental way that batteries generate energy has remained constant. This process involves an electrochemical reaction that takes place within a closed cell. Over 200 years ago, an Italian scientist, named Alessandro Volta, discovered a reliable electrochemical system. However, Volta’s materials and arrangement were inefficient and resulted in a tiny output. Despite this, his work was crucial in laying the foundation for electrochemical advancements. In 1859, Gaston Plante made a significant advancement when he invented a rechargeable battery. Typically, batteries lose their power when their electrodes run out of ions or space to receive them. However, rechargeable batteries are designed so that by applying a charge, the electrodes are either rebuilt or deionized, reversing the primary reaction. In 1859, Gaston Plante made a significant advancement when he invented a rechargeable battery. In 1866, George-Lionel advanced the battery’s evolution by creating a closed, portable, and powerful battery based on Volta’s original concept. This marked the beginning of the consumer battery era, with Leclanche’s invention becoming the first of its kind in the 1890s. His design eventually led to the development of the Duracell alkaline battery in the 1950s. In 1899, Waldemar Jungner made a significant advancement in rechargeable battery technology by combining Leclanche’s concept with new developments in materials science. His battery was smaller in size, more durable, and had a higher output than Plante’s battery, but had less charge capacity. However, due to its high cost, it did not become commercially viable until the 1930s. Throughout most of the twentieth, it served as a battery technology model, with each type fulfilling its own purpose and meeting technology demands. Battery technology underwent gradual development and finally, in 1985, Akira Yoshino created the first commercially viable rechargeable lithium-ion battery. The introduction of lithium-ion batteries in 1992 revolutionized technology. It meant that complex, energy-intensive gadgets like computers and phones could go mobile, and the technology could be upgraded to energy-consuming levels previously impossible. Furthermore, it largely eliminated the problems associated with the unpredictability of renewable energy production by allowing for industrial-scale energy storage.
Batteries can be defined as devices that store energy from external resources through electrochemical processes and provide that energy to other machines, enabling them to operate remotely without requiring a power resource. In recent years, batteries have become increasingly crucial in terms of size and storage capacity [1]. As devices continue to become smaller, batteries are also shrinking in size. As a result, when their unit cells approach molecules and atoms, quantum mechanical effects must be taken into consideration when describing them [2, 3, 4]. Recent theoretical investigations in the field of quantum thermodynamics have demonstrated that entanglement generation is connected to faster work extraction when energy is stored in many-body quantum systems [5]. These findings have prompted research on the use of quantum systems as heat engines and energy storage devices [6]. As a result, there has been a growing interest in the study of quantum batteries, which were first introduced in the influential work by Alicki and Fannes [7]. Scientists are now exploring quantum effects that could potentially enhance the performance of these devices [8, 9, 10, 11]. Quantum batteries are theoretical d-dimensional energy storage quantum systems. They are quantum systems with non-degenerate energy levels that use quantum mechanics principles to store energy [7]. Quantum coherence and entanglement are used in the design of these batteries. Quantum batteries have the potential to be more efficient, have a higher energy density, and be smaller and lighter than classical batteries. The most important feature of a good quantum battery is its ability to store as much energy as possible in the shortest time and discharge it optimally. To determine the quality of a quantum battery, one can examine its internal energy and the amount of work that can be extracted from it [12]. Overall, quantum batteries hold great potential for advancing energy storage technology. Furthermore, progress has been made toward implementing experiments [13, 14, 15, 16, 17].
In the study of quantum batteries, ergotropy is a critical measure that indicates the amount of energy that can be extracted from a given quantum battery state through the cyclic unitary evolutions [12]. The ergotropy of a battery can change from zero, which represents the passive state [18], to a maximum value calculated from the energy levels of the Hamiltonian and the eigenvalues of the battery’s density matrix, as the battery stores or releases energy. Traditionally, quantum mechanics deals with isolated systems that are completely detached from their surrounding environment. The idea of open quantum systems stems from the way physical systems interact with their surroundings. These interactions can cause the transfer of energy, information, or particles between the system and its environment. A major obstacle in studying open quantum systems is decoherence. In quantum physics, decoherence refers to the loss of quantum coherence that arises from the interaction of the quantum system with its environment. Studying quantum batteries from an open quantum systems perspective is crucial as environmental effects on quantum systems are inevitable. There has been extensive research on the impact of environmental parameters on the charging and discharging processes of quantum batteries [19, 20, 21, 22, 23, 24]. When external thermal baths couple with the quantum battery, non-unitary effects may occur, causing energy loss due to thermal effects on the system. In some cases, ergotropy can be stored, but a part of the total energy available in the system cannot be stored as ergotropy. Consequently, part of the total energy cannot be extracted from cyclic unitary evolutions. Therefore, it is important to consider the amount of residual energy that cannot be extracted as useful work from open quantum batteries by unitary processes. To better understand the amount of energy lost during work extraction, it is necessary to examine the constraint of unitary processes. The system exergy represents the maximum amount of work that can be extracted from the system while bringing it into equilibrium with a thermal bath. It can be separated into two parts: ergotropy and residual energy [25]. The residual energy cannot be extracted through a unitary process, and it refers to the non-optimal performance of a cyclic thermodynamics process used to extract work from quantum batteries. Thus, the present chapter describes the relationship between exergy and its potential benefits and effects on the performance of quantum batteries.
2. Energy storage and unitary work extraction
A quantum battery is a type of energy storage device that can be represented by a
with non-degenerate energy levels such that
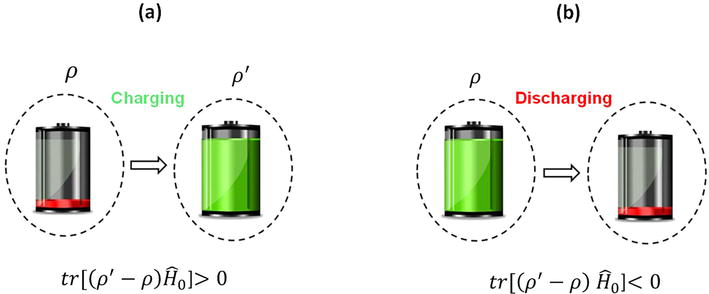
Figure 1.
The time-dependent (a) charging and (b) discharging protocols facilitate the charging and discharging of the battery system through the operation of the Hamiltonian H, which is activated for the duration of the time interval
where
Then, the energy stored in a quantum battery when it’s in state
A practical and intuitive method for constraining the extractable work of Eq. (4) is to examine a thermal state with equivalent entropy to passive state
where the canonical Gibbs state denoted by
The usual scenarios involve the interaction of the quantum system with the external environment, resulting in decoherence and the depletion of quantum resources, thus defining it as an open quantum system. An open quantum battery refers to an open quantum system capable of interacting with the external environment, whether with or without a mediator. As explained earlier, if the battery system is kept isolated, closed, or protected from any external influences, it will always evolve in a unitary manner as described. However, in the case of an open quantum battery, its dynamics are determined by the global Hamiltonian as follows:
where
The super-operator
The implementation of a quantum battery in practice must address the challenge of environmental interactions. As a result, safeguarding against energy leakages and decoherence is crucial for the successful realization of such devices. As a result of these interactions, the entropy level of the battery increases, making unitary evolution insufficient to rectify entropy production and stabilize the system. Notably, the presence of decoherence effects during the charging process negatively impacts the performance of operational quantum batteries, leading to the self-discharge phenomenon [27]. Efforts have been made to mitigate the quantum battery’s interactions with the environment to prevent eventual deactivation, yet certain approaches aim to transform the environment’s role from detrimental to beneficial. Where non-unitary discharging provides more charge due to availability or exergy compared to a cyclic unitary process [25].
3. Exergy in a quantum battery
When the system’s temperature deviates from that of the surrounding environment, it fails to achieve mutual stable equilibrium. Consequently, the requisite conditions for mutual thermal equilibrium are not fulfilled. Any absence of mutual stable equilibrium between a system and its environment can be leveraged to generate work.
In this section, consider work extraction from a quantum system with Hamiltonian
where
The quantity
In other instances, it should be noted that the system may be considered to be in contact with a reservoir that adheres to fermionic (or bosonic) statistics with a chemical potential of
where
3.1 Balance equation
After the extraction of ergotropy, it is established that the internal energy of the system does not diminish to zero [12, 33], thereby leaving a residual amount of energy still accessible within the system. To measure the amount of energy that can be extracted following ergotropy’s extraction, one can analyze the system’s dynamics, as illustrated in Figure 2 [25]. The available work in the initial state
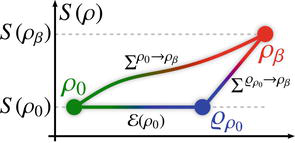
Figure 2.
A visual illustration of the three states under discussion is depicted in the schematic. The extraction of ergotropy, which leaves the system entropy unchanged, is succeeded by the thermalization process, leading the system to reach equilibrium with a thermal reservoir at reverse temperature
where
This outcome has dual interpretations. The initial interpretation concerns the distinctive nature of an energetically efficient starting point for storing ergotropy. When a system interacts with a reservoir, the initial ergotropy is stored in a non-pure state
Here,
4. Conclusions
This chapter addresses the dissipation of energy during cyclic unitary work extraction from a quantum system, with a focus on the identification of this energy loss as the exergy of the quantum passive state. It was demonstrated that, in a real-world scenario, the ergotropy results in energy loss due to the constraints of the unitary process. Additionally, the presence and uniqueness of an optimal passive state for ergotropy and exergy extraction is examined by taking into account the system-bath interaction that leads to thermalization. With respect to the second law of thermodynamics, our key finding was explained as a natural outcome of the entropy production during the thermalization process for exergy extraction. Our results also led to the identification of a range of ergotropy and exergy extraction processes where the total quantum correlations of the system (measured through quantum discord) are conserved. This implies that the exergy of a quantum passive state can be preserved as quantum correlations. As exergy represents the amount of energy recoverable through a thermalization process, our findings open up new possibilities for developing operational protocols for open quantum batteries.
Acknowledgments
This work has been supported by the University of Kurdistan. F.H. Kamin and S. Salimi thank Vice Chancellorship of Research and Technology, University of Kurdistan.
References
- 1.
Scrosati B. Modern Batteries: An Introduction to Electrochemical Power Sources. 2nd ed. London: Arnold; 1997. p. 351 - 2.
Campaioli F, Pollock FA. Vinjanampathy S. In: Binder F, Correa L, Gogolin C, Anders J, Adesso G, editors. Thermodynamics in the Quantum Regime: Fundamental Aspects and New Directions. Cham: Springer; 2018. pp. 207-225. DOI: 10.1007/978-3-319-99046-0_8 - 3.
Goold J, Huber M, Riera A, del Rio L, Skrzypczyk P. The role of quantum information in thermodynamics-a topical review. Journal of Physics A: Mathematical and Theoretical. 2016; 49 :143001. DOI: 10.1088/1751-8113/49/14/143001 - 4.
Vinjanampathy S, Anders J. Quantum thermodynamics. Contemporary Physics. 2016; 57 :545-579. DOI: 10.1080/00107514.2016.1201896 - 5.
Hovhannisyan KV, Perarnau-Llobet M, Huber M, Acín A. Entanglement generation is not necessary for optimal work extraction. Physical Review Letters. 2013; 111 :240401. DOI: 10.1103/PhysRevLett.111.240401 - 6.
Levy UA, Kosloff R. Quantum heat machines equivalence, work extraction beyond Markovianity, and strong coupling via heat exchangers. Entropy. 2016; 18 :124. DOI: 10.3390/e18040124 - 7.
Alicki R, Fannes M. Entanglement boost for extractable work from ensembles of quantum batteries. Physical Review E. 2013; 87 :042123. DOI: 10.1103/PhysRevE.87.042123 - 8.
Campaioli F, Pollock FA, Binder FC, Céleri L, Goold J, Vinjanampathy S, et al. Enhancing the charging power of quantum batteries. Physical Review Letters. 2017; 118 :150601. DOI: 10.1103/PhysRevLett.118.150601 - 9.
Binder FC, Vinjanampathy S, Modi K, Goold J. Quantacell: Powerful charging of quantum batteries. New Journal of Physics. 2015; 17 :075015. DOI: 10.1088/1367-2630/17/7/075015 - 10.
Gyhm J-Y, Šafránek D, Rosa D. Quantum charging advantage cannot Be extensive without global operations. Physical Review Letters. 2022; 128 :140501. DOI: 10.1103/PhysRevLett.128.140501 - 11.
Kamin FH, Tabesh FT, Salimi S, Santos AC. Entanglement, coherence, and charging process of quantum batteries. Physical Review. 2020; 102 :052109. DOI: doi.org/10.1103/PhysRevE.102.052109 - 12.
Allahverdyan AE, Balian R, Nieuwenhuizen TM. Maximal work extraction from finite quantum systems. Europhysics Letters. 2004; 67 :565. DOI: 10.1209/epl/i2004-10101-2 - 13.
Quach JQ, McGhee KE, Ganzer L, Rouse DM, Lovett BW, Gauger EM, et al. Superabsorption in an organic microcavity: Toward a quantum battery. Science Advances. 2022; 8 :eabk3160. DOI: 10.1126/sciadv.abk3160 - 14.
Hu C-K, Qiu J, Souza PJP, Yuan J, Zhou Y, Zhang L, et al. Optimal charging of a superconducting quantum battery. Quantum Science and Technology. 2022; 7 :045018. DOI: 10.1088/2058-9565/ ac8444 - 15.
Joshi J, Mahesh TS. Experimental investigation of a quantum battery using star-topology NMR spin systems. Physical Review A. 2022; 106 :042601. DOI: 10.1103/PhysRevA.106.042601 - 16.
Gemme G, Grossi M, Ferraro D, Vallecorsa S, Sassetti M. IBM quantum platforms: A quantum battery perspective. Batteries. 2022; 8 :2313-0105. DOI: 10.3390/batteries8050043 - 17.
Huang X, Wang K, Xiao L, Gao L, Lin H, Xue P. Demonstration of the charging progress of quantum batteries. Physical Review A. 2023; 107 :L030201. DOI: 10.1103/PhysRevA.107.L030201 - 18.
Pusz W, Woronowicz SL. Passive states and KMS states for general quantum systems. Communications in Mathematical Physics. 1978; 58 :273-290. DOI: 10.1007/BF01614224 - 19.
Ghosh S, Chanda T, Mal S, Sen(De) A. Fast charging of a quantum battery assisted by noise. Physical Review A. 2021; 104 :032207. DOI: 10.1103/PhysRevA.104.032207 - 20.
Kamin FH, Tabesh FT, Salimi S, Kheirandish F, Santos AC. Non-Markovian effects on charging and self-discharging process of quantum batteries. New Journal of Physics. 2020; 22 :083007. DOI: 10.1088/1367-2630/ab9ee2 - 21.
Kamin FH, Abuali Z, Ness H, Salimi S. Quantum battery charging by non-equilibrium steady-state currents. Journal of Physics A: Mathematical and Theoretical. 2023; 56 :275302. DOI: 10.1088/1751-8121/acdb11 - 22.
Barra F. Dissipative charging of a quantum battery. Physical Review Letters. 2019; 122 :210601. DOI: 10.1103/PhysRevLett.122.210601 - 23.
Pirmoradian F, Mølmer K. Aging of a quantum battery. Physical Review A. 2019; 100 :043833. DOI: 10.1103/PhysRevA.100.043833 - 24.
Tabesh FT, Kamin FH, Salimi S. Environment-mediated charging process of quantum batteries. Physical Review A. 2020; 102 :052223. DOI: 10.1103/PhysRevA.102.052223 - 25.
Kamin FH, Salimi S, Santos AC. Exergy of passive states: Waste energy after ergotropy extraction. Physical Review E. 2021; 104 :034134. DOI: 10.1103/PhysRevE.104.034134 - 26.
Breuer HP, Petruccione F. The Theory of Open Quantum Systems. New York: Oxford University Press; 2007. 625 p. DOI: 10.1093/acprof:oso/9780199213900.001.0001 - 27.
Santos AC, Çakmak B, Campbell S, Zinner NT. Stable adiabatic quantum batteries. Physical Review E. 2019; 100 :032107. DOI: 10.1103/PhysRevE.100.032107 - 28.
Vedral V. The role of relative entropy in quantum information theory. Reviews of Modern Physics. 2002; 74 :197. DOI: 10.1103/RevModPhys.74.197 - 29.
Skrzypczyk P, Short AJ, Popescu S. Work extraction and thermodynamics for individual quantum systems. Nature Communications. 2014; 5 :4185. DOI: 10.1038/ncomms5185 - 30.
Parrondo JMR, Horowitz JM, Sagawa T. Thermodynamics of information. Nature Physics. 2015; 11 :131-139. DOI: 10.1038/nphys 3230 - 31.
Rant Z. Ein neues Wort fur technische Arbeits-fahigkeit. Forschung im Ingenieurwesen. 1956; 22 :36-37 - 32.
Schlögl F. Probability and Heat: Fundamentals of Thermostatistics. Wiesbaden: Vieweg+Teubner Verlag Springer; 2013. p. 252 - 33.
Moraes LFC, Saguia A, Santos AC, Sarandy M. Charging power and stability of always-on transitionless driven quantum batteries. EPL. 2021; 136 :23001. DOI: 10.1209/0295-5075/ac1363 - 34.
Alicki R. The quantum open system as a model of the heat engine. Journal of Physics A: Mathematical and General. 1979; 12 :L103. DOI: 10.1088/0305-4470/12/5/007