Abstract
The incidence of incisional hernia after intra-abdominal surgery is approximately 10–15%. Midline incisions are riskier than other incisions. Smoking, surgical site infections, conditions that impair wound healing, and incorrect surgical technique are among the risk factors, especially obesity. It typically presents as swelling on or near the incision. Computed tomography or ultrasonography can be performed for incisional hernias that cannot be detected by physical examination. Preoperative CT scan is important for the surgical strategy, especially for ventral hernias larger than 10 cm and with loss of space. The surgical strategy may vary depending on the size of the hernia. Tension-free repair is accepted as the standard approach by many authors, and suture repair alone is rarely used. The technique of separating into anterior or posterior components can be used in hernias larger than 10 cm with loss of space. Reconstruction using prosthesis material placed preperitoneally (underlay or sublay) is the most commonly used method today.
Keywords
- incisional hernia
- rives–Stoppa repair
- classification
- laparoscopic repair
- component seperation technique
1. Introduction
An incisional hernia is defined as a swelling or abdominal wall cavity in the postoperative incision area that can be detected by physical examination or radiological imaging [1]. Incisional hernia occurs in approximately 10 to 15 percent of patients with a previous abdominal incision [2]. It may develop after midline, mc burney, paramedian, or other abdominal incisions [3, 4]. Its incidence varies according to the incision that has been made. In general, the risk of hernia formation in midline incisions is higher than the other incision types [5, 6, 7]. In addition, the risk of incisional hernia formation is higher in upper abdominal incisions compared to lower abdominal incisions [8, 9, 10, 11]. Both patient-related and technical factors play a role in the development of incisional hernias.
1.1 Patient related factors
Age, obesity, smoking, connective tissue diseases, use of immunosuppressive drugs, malnutrition, diabetes, vascular, and other comorbid diseases are factors that increase the risk of having an incisional hernia. Numerous studies have shown that obesity, in particular, increases the risk of having an incisional hernia, and the rate of postoperative complications and recurrence [12, 13, 14, 15, 16, 17, 18].
1.2 Technical factors
Wound infection, excessive wound tension, and not following the abdominal closure principles predispose to the formation of incisional hernia [19]. The incidence of incisional hernia increases in open bariatric surgery and interventions for repairing abdominal aortic aneurysm.
Abdominal closure should be done effectively, neither tension nor ischemia. In order to achieve this, it is necessary to comply with the appropriate suture material and stitch size. The suture should be the smallest, it can be to be strong enough to hold the wound intact [20]. Suture diameter and its type (monofilament, multifilament, or synthetic) is an effective factor on the amount of foreign matter and bacterial accumulation in the wound. It has been proved that nonabsorbable sutures reduce the risk of wound separation and developing a hernia compared to absorbable sutures. Synthetic nonabsorbable monofilament sutures (polypropylene, etc.) are more resistant to infections than multifilament sutures and natural fibers. Therefore, suture composition and structure affect the rate of bacterial absorption and proliferation [20]. Some studies have reported that sutures coated with antimicrobial compounds can reduce surgical site infection rates [21, 22, 23, 24, 25, 26, 27, 28, 29]. However, in other studies, whether the suture was covered or uncoated with antimicrobial compounds did not differ in terms of surgical site infection [25].
The method of closing the abdominal wall is another factor that affects developing an incisional hernia. While stratified closure is defined as the closure of individual components of the abdominal wall (peritoneum, muscular and aponeurotic structures, subcutaneous adipose tissue), mass closure is the closure of other abdominal wall layers as a single structure excluding the skin [30, 31, 32]. Studies have shown that mass closure is associated with a lower incidence of developing an incisional hernia [28, 29, 33]. Other than this, it has been shown that intermittent and uninterrupted closures have different effects on the tension of the wound and perfusion of the tissue. The amount of the suture used depends on the size of each suture (depends on the distance from fascial edge) and gap between sutures. For continuous closure, the total length of the suture should be about four times the incision length [31, 32]. In a randomized study, hernia recurrence was shown to be decreased if the ratio of 1/4 was adhered to [34]. The suture should be placed approximately about 1 cm from the edge of the fascia. The 2015 guidelines of the European Hernia Society recommend reducing the suture width from 10 mm to about 5 to 8 mm [31, 32, 33, 34, 35, 36]. In a randomized study, it was shown that longer suture width increases the risk of developing both incisional hernia and surgical site infection [32]. Studies have shown that the most appropriate method for abdominal wall closure is continuous mass closure using slowly absorbable sutures with a 4:1 ratio of suture length to wound length [37, 38, 39].
2. Classification
Incisional hernias can be classified as anatomically and clinically.
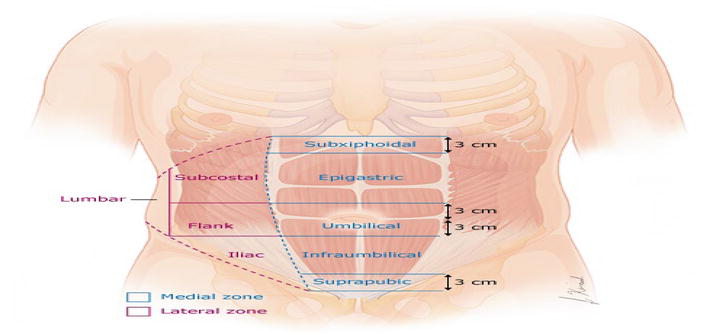
Figure 1.
The European hernia society (EHS) classification for incisional abdominal wall hernias divides the abdomen into a medial zone and a lateral zone. The medial zone, defined as medial to the lateral margin of the rectus sheath, is subdivided into five subzones (subxiphoid, epigastric, umbilical, infraumbilical, and suprapubic). The lateral zone is subdivided into four subzones (subcostal, flank, iliac, and lumbar).
According to size, incisional hernias are classified into three categories: <1 cm, 1 to 10 cm, and > 10 cm [40]. Hernias larger than 10 cm in width are defined as complex or giant ventral hernias. These require additional preparation [41].
An incarcerated hernia is a hernia that its contents cannot be reduced to the abdomen due to a narrow opening in the abdominal wall facial defect or adhesions between the contents and the hernia sac [42]. An incarcerated hernia containing an intestinal loop can cause intestinal obstruction [43]. In a population study of more than 23,000 patients followed without surgery, a cumulative rate of incarceration of incisional hernias was reported 1.24% in 1 year and 2.59% in 5 years [44].
The degree of domain loss has been calculated differently by various authors. When the ratio of hernia sac volume to residual abdominopelvic cavity volume is calculated and if it is 25% and above, preoperative abdominal expansion is required [45]. When the ratio of the hernia sac volume to the entire peritoneal volume is calculated and if it is higher than 20%, it predicts how hard the closure will be [46]. Hernia reduction may lead to serious complications such as persistent hypertension in the abdominopelvic cavity, abdominal compartment syndrome due to visceral edema and postoperative fluid resuscitation.
3. Management
3.1 Acute incarcerated or strangulated hernias
Acute incarcerated or strangulated incisional hernias require immediate surgical repair (Figure 2). The aim of emergency surgery is to resolve the acute problem (intestinal necrosis or obstruction) and to perform a safe and durable repair. The optimal technique of hernia repair depends on the patient’s anatomy, stability, comorbidities, and the degree of operative site contamination. Once the source of the contamination is controlled, surgical repair of the hernia can be done only with suture repair, mesh repair, or incremental repair (placement of absorbable mesh and leaving the hernia to be repaired electively). There is no consensus because the clinical situation is heterogeneous [47].
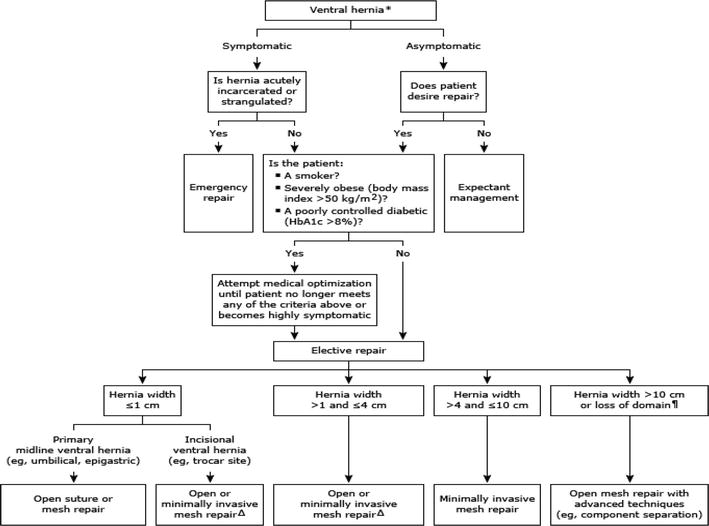
Figure 2.
Management of ventral hernias.
The best practice for a ventral hernia in a contaminated area is safe and, whenever possible, suture-only or incremental repair. Hernia repair with synthetic mesh has been proved to be safe and effective in the hands of powerful surgeons in these complex cases [48]. The role of biological and biosynthetic networks has not yet been defined.
3.2 Surgical treatment of incisional hernias
Underlay meshes are used on standard laparoscopic repairs. Although it has advantages such as less pain, healing fast, and having lower infection rate, swelling continues at the defect site when intra-abdominal pressure increases. Repair of large defects with mesh has been shown not to reduce abdominal and back pain and respiratory problems when compared to component separation, which restores the dynamic muscle component to the abdominal wall.
In patients with large incisional hernias and patients with space gap, we recommend using advanced techniques such as component separation to achieve primary fascial closure prior to mesh augmentation, rather than using inlay mesh to bridge a facial defect.
The decomposition, pioneered by Ramirez [56], is used to repair the large and complex midline abdominal wall defects and has the advantage of restoring abdominal wall function. Component separation involves dividing the anterior or posterior rectus sheaths and/or portions of the lateral oblique muscle to allow the rectus abdominis muscle to advance by about 10 cm from each side to allow a fascial closure under physiological tension [56, 57, 58].
The decision to separate its components is made based on physical examination and cross-sectional imaging. Depending on the width of the hernia and the surgeon’s experience and preference, any dissection technique can be chosen.
Typically, hernias less than 7 cm do not require a component separation (Figure 3) and can usually be repaired with an open, laparoscopic, or robotic approach with intraperitoneal mesh, or with an open or robotic approach with retrorectus mesh, as described by Rives and Stoppa. Such hernias can also be repaired using open techniques with onlay mesh [58].
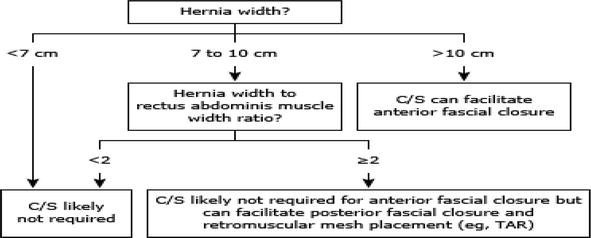
Figure 3.
Determining the need for component separation in ventral hernia repair.
Hernias that are 7 to 10 cm typically do not require a component separation for the midline facial approach and can be repaired laparoscopically with a large piece of intraperitoneal mesh or with an open retromuscular approach. If necessary, an open or robotic TAR can be added to ensure closure of the posterior rectus sheath. A ratio of rectus width to hernia width > 2 reliably predicts that the fascia can be closed with a Rives–Stoppa repair alone, with no further myofascial loosening in nearly 90% of cases [59].
Hernias larger than 10 cm might require separation of the anterior or posterior component for both the rectus approach (midline closure) and posterior facial approach, depending on the compliance of the abdominal wall. This can be accomplished with open anterior component separation or open or robotic TAR.
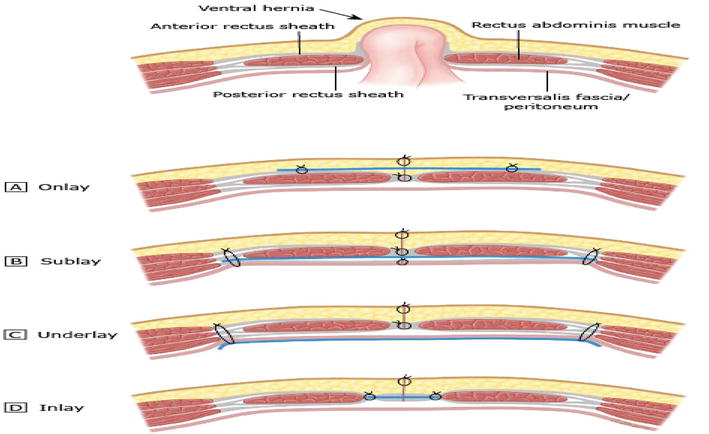
Figure 4.
Mesh locations: (A) onlay, (B) sublay, (C) underlay, and (D) inlay. The blue line depicts mesh location.
Randomized studies have proved that both recurrence and complication rates were higher with onlay andinlay meshes than with sublay and underlay meshes [61].
4. Open anterior component separation technique
Posterior rectus sheath is cut 1 to 2 cm from lateral to the medial border of the rectus muscle, starting from the costal margin and continuing below the arcuate line where the posterior sheath becomes continuous with the peritoneum and transversalis fascia. Next, the external oblig muscles should be divided. It is made through an incision about 1 to 2 cm length from lateral of the linea semilunaris. The external oblig muscle is not always divided in its entire length; instead, the length of the cleavage can be adjusted according to the location of the facial defect in the midline.
Releasing the underlying internal oblig/transversus abdominis muscle apparatus from the divided external oblig muscle reveals an avascular plane. The midline facial approach should be attempted after each myofascial relaxation, not after the division of both the posterior rectus sheaths and the EO muscles. Further myofascial relaxation should be avoided after a tension-free midline approach that has been achieved. After adequate myofascial relaxation is achieved, the fascia should be closed with a slowly absorbable monofilament suture. After a sufficiently large mesh is fixed to the external oblig muscle edge, an absorbent drain is placed on the mesh and the skin is closed (Figure 5).
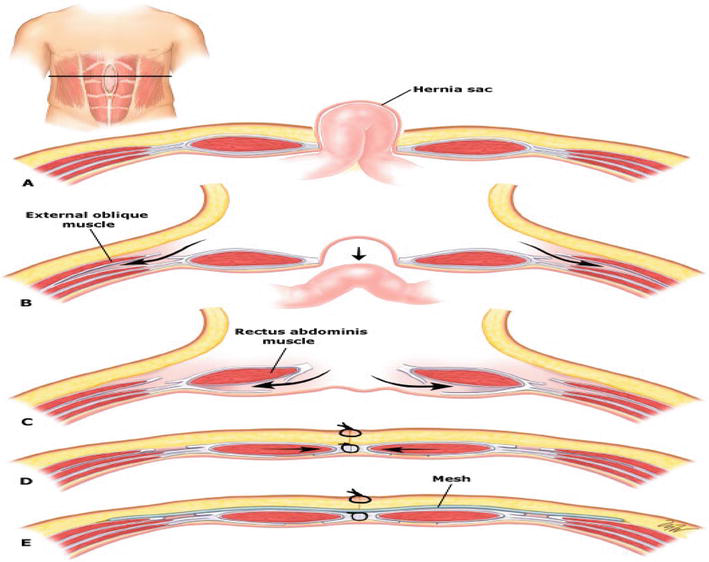
Figure 5.
Component seperation technique: A(Hernia sac), B(external oblig muscles be divided), C(Posterior rectus sheath is cut 1 to 2 cms from lateral to the medial border of the rectus muscle), D(After adequate myofascial relaxation is achieved, the fascia should be closed with a slowly absorbable monofilament suture), E(mesh location).
5. Separation technique to open posterior components (rives: stoppa retrorectus dissection and transverse abdominis release (TAR))
The operation begins with a large midline laparotomy that includes the patient’s previous scar. During myofascial dissection, intra-abdominal adhesions are completely dissolved and the internal organs are released from the abdominal wall and a wide towel is laid on the intestine. After the towel is placed, the edges of the facial defect are determined and the length/width is measured.
Five Kocher clamps are then placed on the medial edge of a rectus. Using a toothed forceps, the posterior rectus sheath is retracted and cut just beside the rectus margin to expose the underlying muscle hub. The entire medial edge of the posterior rectus sheath should be separated and the medial edge of the rectus muscle should be exposed. The retrorectus area is developed using firm but gentle traction on the anterior and posterior rectus sheaths and cautery to control small epigastric perforators. The lateral extension of this space is marked by large neurovascular bundles and deep lower epigastric vessels, especially in the upper and lower directions of this dissection, before the vessels progress toward the rectus muscle hub. Typically, there is one large medial neurovascular perforator that can be sacrificed in the superior third of the retrorectus space, but the remainder should be preserved if possible. Neurovascular bundles define the lateral extension of the retrorectus space, and their preservation prevents accidental division of the lineasemilunaris, a devastating complication of this technique. At this point, if the posterior rectus sheath is sufficiently medialized to the midline and the contralateral dissection allows isolation of the internal organs, then the Rives–Stoppa dissection alone is sufficient. If the release is not sufficient until this stage, transverse abdominis release is started. The posterior lamella of the internal oblique (IO) and transversus abdominis (TA) muscle departs just from medial to the neurovascular bundles marking the lineasemilunaris. After the transversusabdominis muscle is completely separated from the transversus fascia from cranial to caudal, the posterior rectus sheath is reapproximated and the mesh is placed on it and fixed. After the anterior rectus is approached in the sheath, a drain is placed and the skin is closed (Figure 6).
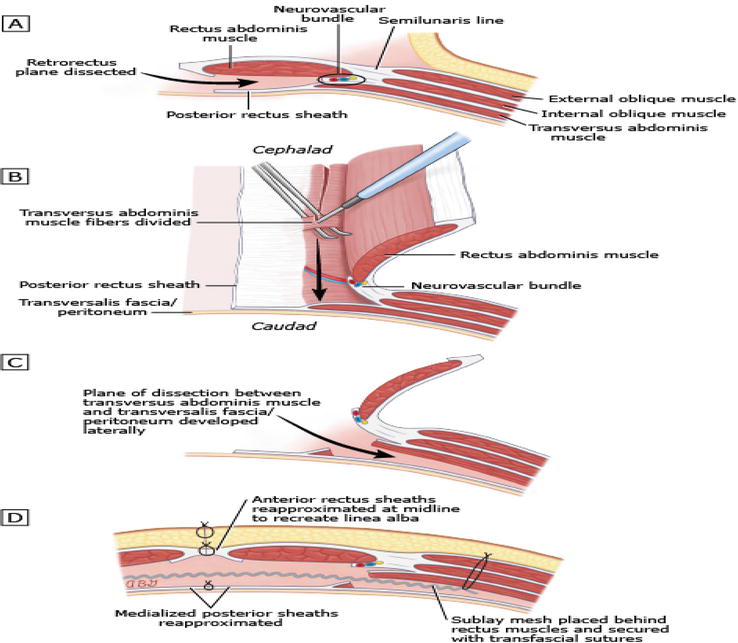
Figure 6.
Transversus abdominis release (TAR): A(Retrorectus plane dissected), B(Transversus Abdominis muscle fibers divided), C(Plane of dissection), D(Sublay mesh placed).
Anterior component separations should not be performed in conjunction with posterior component separation (e.g., TAR) as this might significantly destabilize the abdominal wall and cause permanent disability.
Separation into both anterior and posterior components can also be done laparoscopically or robotically.
6. Presurgery assistants
Preoperative adjuvants (such as botulinum toxin injection or tissue expansion) were used to facilitate fascial and/or abdominal wall closure when the ratio of hernia volume to peritoneal volume was above 20–25%.
There is no consensus on which patients would benefit from BoNT-A injections prior to ventral hernia repair. However, many authors indicate that preoperative BoNT-A can be used in patients with a hernia volume to peritoneal volume ratio > 20 to 25% [64]. This should be done at least 2 weeks before attempting ventral hernia repair.
References
- 1.
Korenkov M, Paul A, Sauerland S, et al. Classification and surgical treatment of incisional hernia. Langenbeck's Archives of Surgery. 2001; 386 :65-73 - 2.
Nachiappan S, Markar S, Karthikesaligam A, et al. Prophylactic mesh placement in high-risk patients undergoing elective laparotomy: A systematic review. World Journal of Surgery. 2013; 37 :1861-1871 - 3.
Mudge M, Hughes LE. Incisional hernia: A 10 year prospective study of incidence and attitudes. British Journal of Surgery. 1985; 72 (1):70-71 - 4.
Kingsnorth A, LeBlanc K. Hernias: Inguinal and incisional. The Lancet. 2003; 362 (9395):1561-1571 - 5.
Bucknall TE, Cox PJ, Ellis H. Burst abdomen and incisional hernia: A prospective study of 1129 major laparotomy. British Medical Journal (Clinical Research Ed.). 1982; 284 (6320):931-933 - 6.
SSanders DL, Kingsnorth AN. The modern management of incisional hernias. BMJ. 9 May 2012; 344 :e2843. DOI: 10.1136/bmj.e2843. PMID: 22573647 - 7.
Bickenbach KA et al. Up and down or side to side? A systematic review and meta-analysis examining the impact of incision on outcomes after abdominal surgery. The American Journal of Surgery. 2013; 206 (3):400-409 - 8.
Fassiadis N et al. Randomized clinical trial of vertical or transverse laparotomy for abdominal aortic aneurysm repair. Journal of British Surgery. 2005; 92 (10):1208-1211 - 9.
Inaba T et al. Prospective randomized study of two laparotomy incisions for gastrectomy: Midline incision versus transverse incision. Gastric Cancer. 2004; 7 :167-171 - 10.
Levrant SG, Bieber E, Barnes R. Risk of anterior abdominal wall adhesions increases with number and type of previous laparotomy. The Journal of the American Association of Gynecologic Laparoscopists. 1994; 1.4 (Part 2):S19-S19 - 11.
Brown SR, Goodfellow PB. Transverse verses midline incisions for abdominal surgery. Cochrane Database Syst Rev. 19 Oct 2005; 2005 (4):CD005199. DOI: 10.1002/14651858.CD005199.pub2. PMID: 16235395; PMCID: PMC8866010 - 12.
Itatsu K, Yokoyama Y, Sugawara G, et al. Incidence of and risk factors for incisional hernia after abdominal surgery. The British Journal of Surgery. 2014; 101 :1439 - 13.
George CD, Ellis H. The results of incisional hernia repair: A twelve year review. Ann R Coll Surg Engl. Jul 1986; 68 (4):185-187. PMID: 3789602; PMCID: PMC2498374 - 14.
Bosanquet DC, Ansell J, Abdelrahman T, et al. Systematic review and meta-regression of factors affecting midline incisional hernia rates: Analysis of 14,618 patients. PLoS One. 2015; 10 :e0138745 - 15.
Lau B, Kim H, Haigh PI, Tejirian T. Obesity increases the odds of acquiring and incarcerating noninguinal abdominal wall hernias. The American surgeon. 2012; 78 :1118 - 16.
Holihan JL, Alawadi Z, Martindale RG, et al. Adverse events after ventral hernia repair: The vicious cycle of complications. Journal of the American College of Surgeons. 2015; 221 :478 - 17.
Berger RL, Li LT, Hicks SC, et al. Development and validation of a risk-stratification score for surgical site occurrence and surgical site infection after open ventral hernia repair. Journal of the American College of Surgeons. 2013; 217 :974 - 18.
Liang MK, Goodenough CJ, Martindale RG, et al. External validation of the ventral hernia risk score for prediction of surgical site infections. Surgical Infections. 2015; 16 :36 - 19.
Seiler CM, Deckert A, Diener MK, et al. Midline versus transverse incision in major abdominal surgery: A randomized, double-blind equivalence trial (POVATI: ISRCTN60734227). Annals of Surgery. 2009; 249 :913 - 20.
Yaltirik M, Dedeoglu K, Bilgic B, et al. Comparison of four different suture materials in soft tissues of rats. Oral Diseases. 2003; 9 :284 - 21.
McGeehan D, Hunt D, Chaudhuri A, Rutter P. An experimental study of the relationship between synergistic wound sepsis and suture materials. The British Journal of Surgery. 1980; 67 :636 - 22.
Alexander JW, Kaplan JZ, Altemeier WA. Role of suture materials in the development of wound infection. Annals of Surgery. 1967; 165 :192 - 23.
Justinger C, Moussavian MR, Schlueter C, et al. Antibacterial [corrected] coating of abdominal closure sutures and wound infection. Surgery. 2009; 145 :330 - 24.
Nakamura T, Kashimura N, Noji T, et al. Triclosan-coated sutures reduce the incidence of wound infections and the costs after colorectal surgery: A randomized controlled trial. Surgery. 2013; 153 :576 - 25.
Diener MK, Knebel P, Kieser M, et al. Effectiveness of triclosan-coated PDS plus versus uncoated PDS II sutures for prevention of surgical site infection after abdominal wall closure: The randomized controlled PROUD trial. Lancet. 2014; 384 :142 - 26.
Nordkam RA, Bluyssen SJ, van Goor H. Randomized clinical trial comparing blunt tapered and standard needles in closing abdominal fascia. World Journal of Surgery. 2005; 29 :441 - 27.
Iwase K, Higaki J, Tanaka Y, et al. Running closure of clean and contaminated abdominal wounds using a syntheticfilament absorbable looped suture. Surgery Today. 1999; 29 :874 - 28.
Rucinski J, Margolis M, Panagopoulos G, Wise L. Closure of the abdominal midline fascia: Meta-analysis delineates the optimal technique. The American Surgeon. 2001; 67 :421 - 29.
Ceydeli A, Rucinski J, Wise L. Finding the best abdominal closure: An evidence-based review of the literature. Current Surgery. 2005; 62 :220 - 30.
Gislason H, Grønbech JE, Søreide O. Burst abdomen and incisional hernia after major gastrointestinal operations--comparison of three closure techniques. The European Journal of Surgery= Actachirurgica. 1995; 161 (5):349-354 - 31.
Muysoms FE, Antoniou SA, Bury K, et al. European hernia society guidelines on the closure of abdominal wall incisions. Hernia. 2015; 19 :1 - 32.
Millbourn D, Cengiz Y, Israelsson LA. Effect of stitch length on wound complications after closure of midline incisions: A randomized controlled trial. Archives of Surgery. 2009; 144 :1056 - 33.
van’t Riet M, Steyerberg EW, Nellensteyn J, et al. Meta-analysis of techniques for closure of midline abdominal incisions. The British Journal of Surgery. 2002; 89 :1350 - 34.
Israelsson LA, Jonsson T. Closure of midline laparotomy incisions with polydioxanone and nylon: The importance of suture technique. The British Journal of Surgery. 1994; 81 :1606 - 35.
Cengiz Y, Gislason H, Svanes K, Israelsson LA. Mass closure technique: An experimental study on separation of wound edge. The European Journal of Surgery. 2001; 167 :60 - 36.
Deerenberg EB, Harlaar JJ, Steyerberg EW, et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): A double-blind, multicentre, randomized controlled trial. Lancet. 2015; 386 :1254 - 37.
Seid MH, McDaniel-Owens LM, Poole GV Jr, Meeks GR. A randomized trial of abdominal incision suture technique and wound strength in rats. Archives of Surgery. 1995; 130 :394 - 38.
Meeks GR, Nelson KC, Byars RW. Wound strength in abdominal incisions: A comparison of two continuous mass closure techniques in rats. American Journal of Obstetrics and Gynecology. 1995; 173 :1676 - 39.
Colombo M, Maggioni A, Parma G, et al. A randomized comparison of continuous versus interrupted mass closure of midline incisions in patients with gynecologic cancer. Obstetrics and Gynecology. 1997; 89 :684 - 40.
Muysoms FE, Miserez M, Berrevoet F, et al. Classification of primary and incisional abdominal wall hernias. Hernia. 2009; 13 :407 - 41.
Halligan S, Parker SG, Plumb AA, Windsor ACJ. Imaging complex ventral hernias, their surgical repair, and their complications. European Radiology. 2018; 28 :3560 - 42.
Birendelli A, Sartelli M, Di Saverio S, et al. 2017 update of the WSES guidelines for emergency repair of complicated abdominal wall hernias. World Journal of Emergency Surgery. 2017; 12 :37 - 43.
Courtney CA, Lee AC, Wilson C, O'Dwyer PJ. Ventral hernia repair: A study of current practice. Hernia. 2003; 7 :44 - 44.
Dadashzadeh ER, Huckaby LV, Handzel R, et al. The risk of incarceration during nonoperative Management of Incisional Hernias: A population-based analysis of 30,998 patients. Annals of Surgery. 2022; 275 :e488 - 45.
Tanaka EY, Yoo JH, Rodrigues AJ Jr, et al. A computerized tomography scan method for calculating the hernia sac and abdominal cavity volume in complex large incisional hernia with loss of domain. Hernia. 2010; 14 :63 - 46.
Sabbagh C, Dumont F, Robert B, et al. Peritoneal volume is predictive of tension-free fascia closure of large incisional hernias with loss of domain: A prospective study. Hernia. 2011; 15 :559 - 47.
Bondre IL, Holihan JL, Askenasy EP, et al. Suture, synthetic, or biologic in contaminated ventral hernia repair. The Journal of Surgical Research. 2016; 200 :488 - 48.
Harris HW, Primus F, Young C, et al. Preventing recurrence in clean and contaminated hernias using biologic versus synthetic mesh in ventral hernia repair: The PRICE randomized clinical trial. Annals of Surgery. 2021; 273 :648 - 49.
Liang MK, Holihan JL, Itani K, Alawadi ZM, Gonzalez JR, Askenasy EP, et al. Ventral Hernia Management: Expert Consensus Guided by Systematic Review. Ann Surg. Jan 2017; 265 (1):80-89. DOI: 10.1097/SLA.0000000000001701. PMID: 28009730 - 50.
Kokotovic D, Sjølander H, Gögenur I, Helgstrand F. Watchful waiting as a treatment strategy for patients with a ventral hernia appears to be safe. Hernia. 2016; 20 :281 - 51.
Holihan JL, Alawadi ZM, Harris JW, et al. Ventral hernia: Patient selection, treatment, and management. Current Problems in Surgery. 2016; 53 :307 - 52.
Martin AC, Lyons NB, Bernardi K, et al. Expectant Management of Patients with ventral hernias: 3 years of follow-up. World Journal of Surgery. 2020; 44 :2572 - 53.
Delaney LD, Howard R, Palazzolo K, et al. Outcomes of a Presurgical optimization program for elective hernia repairs among high-risk patients. JAMA Network Open. 2021; 4 :e2130016 - 54.
Frey S, Jurczak F, Fromont G, et al. Are the relative benefits of open versus laparoscopic intraperitoneal mesh repair of umbilical hernias dependent on the diameter of the defect? Surgery. 2022; 171 :419 - 55.
Bikhchandani J, Fitzgibbons RJ Jr. Repair of giant ventral hernias. Advances in Surgery. 2013; 47 :1 - 56.
Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: An anatomical and clinical study. Journal of Plastic, Reconstructive & Aesthetic Surgery. 1990; 86 :519 - 57.
de VriesReilingh TS, van Goor H, Rosman C, et al. “Components separation technique” for the repair of large abdominal wall hernias. Journal of the American College of Surgeons. 2003; 196 :32 - 58.
Rosen MJ, Williams C, Jin J, et al. Laparoscopic versus open-component separation: A comparative analysis in a porcine model. American Journal of Surgery. 2007; 194 :385 - 59.
Love MW, Warren JA, Davis S, et al. Computed tomography imaging in ventral hernia repair: Can we predict the need for myofascial release? Hernia. 2021; 25 :471 - 60.
Parker SG, Halligan S, Liang MK, et al. International classification of abdominal wall planes (ICAP) to describe mesh insertion for ventral hernia repair. The British Journal of Surgery. 2020; 107 :209 - 61.
Albino FP, Patel KM, Nahabedian MY, et al. Does mesh location matter in abdominal wall reconstruction? A systematic review of the literature and a summary of recommendations. Plastic and Reconstructive Surgery. 2013; 132 :1295 - 62.
Bittner R, Bingener-Casey J, Dietz U, et al. Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society [IEHS])—Part 2. Surgical Endoscopy. 2014; 28 :353 - 63.
Ibarra-Hurtado TR, Nuño-Guzmán CM, Echeagaray-Herrera JE, et al. Use of botulinum toxin type a before abdominal wall hernia reconstruction. World Journal of Surgery. 2009; 33 :2553 - 64.
Afaque MY. Assessing the complexity of ventral hernia by methods of Tanaka, Sabbagh, Carbonell, and Love. Hernia. 2021; 25 :557 - 65.
Altubaiti G, Butler CE. Abdominal wall and chest wall reconstruction. Plastic and Reconstructive Surgery. 2014; 133 :688e - 66.
Paletta CE, Huang DB, Dehghan K, Kelly C. The use of tissue expanders in staged abdominal wall reconstruction. Annals of Plastic Surgery. 1999; 42 :259