Abstract
One of the most common surgical cures for lumbar degenerative illnesses is lumbar fusion. Traditional open lumbar fusion is often used in clinical settings and has positive clinical results. However, there are some disadvantages of the traditional open approach, such as tremendous surgical invasiveness and a high risk of complications in the perioperative period. The gold standard for minimally invasive surgical techniques in recent years has been minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF). With the advancement of full-endoscopic spine surgical techniques, endoscopic lumbar surgery has rapidly developed from simple discectomy to decompression of spinal stenosis. Currently, the endoscopic lumbar interbody fusion procedure has been performed. On the basis of adequate spinal canal decompression and dependable interbody fusion, endoscopic lumbar interbody fusion could reduce surgical invasiveness and improve patient recovery. In this chapter, we will give a brief introduction to the advance in endoscopic transforaminal lumbar interbody fusion, focusing on the indication, technical aspects, clinical effectiveness, safety, fusion devices, and novel techniques that could be applied in the near future.
Keywords
- endoscopic
- transforaminal lumbar interbody fusion
- percutaneous
- minimally invasive
- biportal
1. Introduction
Since the first description of endoscopic transforaminal lumbar interbody fusion (TLIF) by Leu et al. in 1997 [1], this technique has undergone more than 20 years of evolution. Concerns have centered on the efficacy and safety of endoscopic TLIF for neural decompression and solid interbody fusion. Numerous studies have demonstrated the efficacy of endoscopic TLIF in relieving low back and leg pain and enhancing the quality of life [2, 3]. Some research has also reported a high incidence of surgical complications, such as nerve root injury, cage migration, and pseudarthrosis, which have been the obstacles to the further development of endoscopic TLIF [4].
Surgeons from all over the world have conducted valuable explorations, including the innovations of endoscopic instruments and improvements in surgical technique. These advancements have increased the effectiveness and safety of endoscopic TLIF, allowing it to be applied as a routine procedure of minimally invasive spine surgery.
In this chapter, we will give a brief introduction to the advance in endoscopic TLIF, focusing on the indication, technical aspects, clinical effectiveness, safety, fusion devices, and novel techniques that could be applied in the near future.
2. Surgical Indications and Stepwise selection
2.1 Surgical indications
Discogenic low back pain, lumbar foraminal or lateral recess stenosis with segmental instability, and Meyerding Grade I-II degenerative/isthmic spondylolisthesis should be the indications for endoscopic TLIF.
2.2 Stepwise selection of indications
According to the technical demanding level, the risks of complications, the controllability of operating time, and the surgeon's proficiency in endoscopic techniques, a stepwise selection of indications for endoscopic TLIF should be considered.
Single-level and unilateral diseases that do not require radical nerve decompression, such as discogenic low back pain, lumbar segmental instability, Meyerding Grade I lumbar spondylolisthesis, and unilateral symptomatic lumbar lateral recess stenosis, should be selected in the early stages of implementing endoscopic TLIF.
Surgeons with sufficient experience in endoscopic decompression and fusion techniques could choose to treat lumbar disc herniation or lumbar spinal stenosis requiring unilateral decompression at double segments or bilateral decompression at single segment, as well as Meyerding grade II lumbar spondylolisthesis.
After proficiently mastering endoscopic fusion techniques, surgeons could choose more challenging lumbar diseases, such as revision surgery for postendoscopic decompression.
For lumbar ossification-related disorders with cauda equina syndrome or revision surgery for postoperative segmental instability, endoscopic TLIF should be performed with caution. However, as endoscopic techniques and instruments continue to advance, the indications for endoscopic TLIF may be expanded.
3. Technical aspects
3.1 Percutaneous endoscopic transforaminal lumbar interbody fusion
Percutaneous endoscopic transforaminal lumbar interbody fusion (PE-TLIF) is performed while the patient is prone under general anesthesia or low-dose epidural anesthesia combined with local anesthesia. Using C-arm fluoroscopy, the lumbar segment is validated. After placing the functional conduit through Kambin's triangle, the endoscope system is connected. Under endoscopic observation, the ligament flavum is dissected, and the superior articular process (SAP) is excised with micro scissors or a burr drill. Then, the lateral spinal canal is decompressed, and the nerve root that traverses the canal is released. Endoscopic confirmation that the traversing and exiting nerve roots are protected from the working channel is followed by the discectomy and removal of the cartilaginous endplates. After the endplates have been sufficiently prepared, the endoscope is removed and the 7 mm PEEK or titanium expandable fusion cage is inserted through the working channel under radiography. The endoscope is used to examine the spinal canal and foramina to ensure that the nerve root is relieved. Using the radioscopic device, four pedicle screws are percutaneously implanted into the predetermined locations. Inserting two rods and tightening the screw-rod attachment. The epidermis is sutured, and the position of the fasteners and cage is re-evaluated using a C-arm fluoroscope.
3.2 Surgical innovations in PE-TLIF contributed by the authors
3.2.1 Safe removal of the superior articular process
The authors innovated in the safe removal of the SAP, as an innovative concept [5, 6]. In brief, syringe needles are used to identify the pedicle positions. Four incisions (5 mm) are made and then 4 primary guide pins are inserted. The depth is determined by fluoroscopy. The inferior primary guide on the hypothetically symptomatic side is confirmed as the first guide pin. At the end of the first guide pin, the specially designed oriented SAP resection device is installed. With the aid of this device, a secondary guide pin is percutaneously inserted into the SAP (Figure 1). After removing the guide, a 12-mm skin incision is made along the secondary guide pin. Dilating and protection cannulas are inserted progressively with the help of a secondary guide pin. While soft tissues and nerves are protected by the protection cannula, the part of the SAP is excised and taken out using a ring saw (Figure 2).
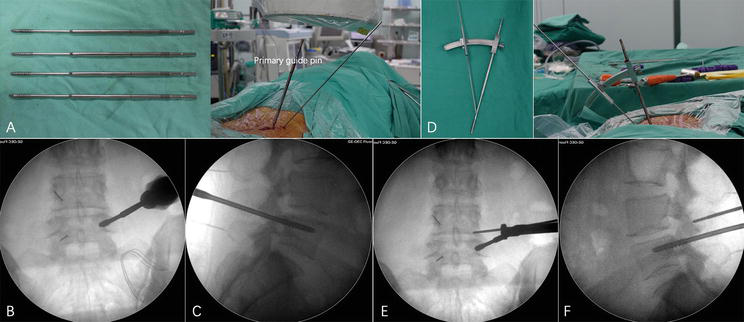
Figure 1.
Guide pin insertion under fluoroscopy. (a) The primary guide pin (left), whose front end is threaded and can be firmly fixed in the pedicle, and its position are readily identifiable under fluoroscopy; the primary guide pin is percutaneously inserted into the vertebral pedicle and rotated to fix (right). (b–c) C-arm anteroposterior and lateral fluoroscopy confirms that the primary guide pin penetrates the pedicle and that the upper thread edge is below the dorsal lateral level of the superior articular process. (d) Physical view of the specially designed SAP guider; the first and second guide pins are connected by a connecting arch, and the angle and depth of the second guide pin's perforation can be adjusted on the connecting arch. (e–f) C-arm anteroposterior and lateral fluoroscopy confirms that the second guide pin is attached to the superior articular process's posterior aspect. Reproduction permission was acquired from Yin et al. [
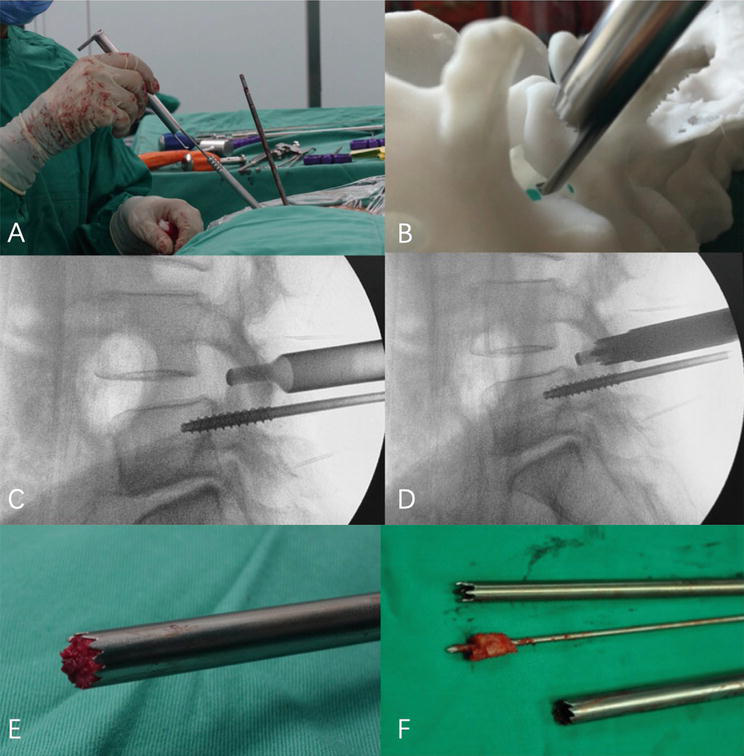
Figure 2.
A-F: Resection method of the superior articular process: the hook-shaped protective sleeve clings to the lateral cortex of the superior articular process, reaches the ventral side of the articular process, protects the exiting nerve root and can control the cutting depth of the trephine at the same time, protects the dura mater and nerve root, and rotates the trephine to remove the superior articular process.
The primary benefit of this method is its ability to safely and effectively resect SAP. The design of the oriented SAP resection device is based on the relatively constant anatomical relationship between SAP and pedicles in the lumbar spine, allowing the removal of a portion of SAP without nerve damage when the standard procedure is followed.
In the meantime, the hook-shaped device in front of the cannula for SAP resection could limit the depth of incision, thereby preventing trepan-cutting of the nerve root and dura mater.
3.2.2 Innovative surgical instruments for intervertebral space handling
The authors developed a set of innovative surgical instruments for intervertebral space handling, including a width-adjustable intervertebral chisel and various nerve-protecting sheaths. These tools could assist the surgeon in precisely placing surgical instruments and endoscopes into the intervertebral space, facilitating safer and more precise operations (Figure 3).
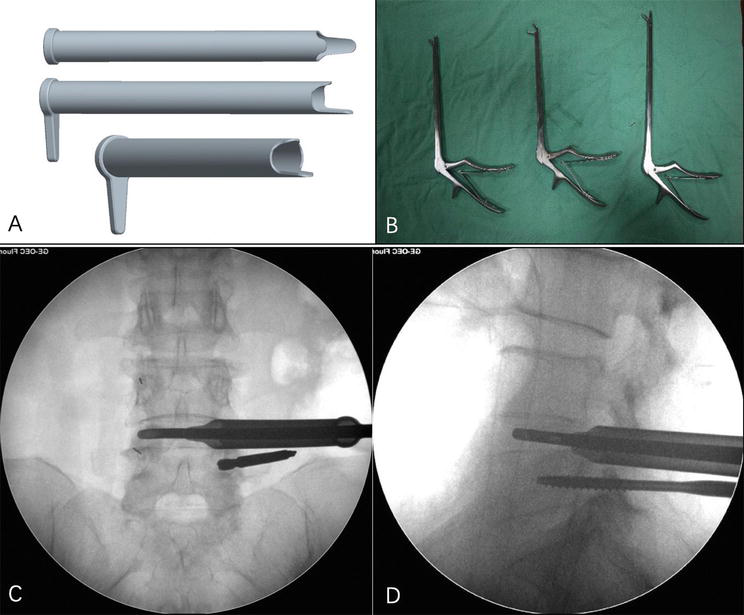
Figure 3.
Intervertebral space handling by the working sleeve. (a) Bone grafting channel with double tongue-shaped nerve-protecting sheaths. The tongue flaps are at the front end, one is long and the other is short; the tongue flap is designed according to the working sleeve tilted at 45°; the long tongue flap is used to protect the dura and nerve root in the spinal canal; and the short tongue flap is used to protect the exit nerve roots; they could simultaneously protect the traversing nerve root and the exiting nerve root outside the bone grafting channel. (b) The 4.5 mm straight and elbow rongeur are used to remove disc tissue. (c–d) Rongeur is used to remove the intervertebral disc tissue and confirm the position of the rongeur to ensure adequate resection of the disc.
3.2.3 Height-adjustable interbody fusion cage
The authors designed a height-adjustable interbody fusion cage, which could withstand greater motion and compressive loads, with its functionality remaining normal even under a 3000N compressive force [5]. Although its implantation channel requires an 8 mm working channel, larger than the B-Twin intervertebral fusion cage's minimum 5 mm channel, the round front end of the adjustable intervertebral fusion cage is more prone to be implanted. The maximal height of 13 mm after expansion could enhance the tension of the longitudinal ligament and annulus fibrosus while decreasing the risk of vertebral body fracture due to excessive expansion. The adjustable intervertebral fusion cage is designed with the characteristics of parallel expansion and a 3°~8° lordosis angle, which could not only consist with the stability of the superior/inferior endplates but also meet the sagittal lordosis requirement of lumbar spine. Moreover, its tapered sawtooth cross-section can reduce the risk of fusion cage migration (Figure 4).
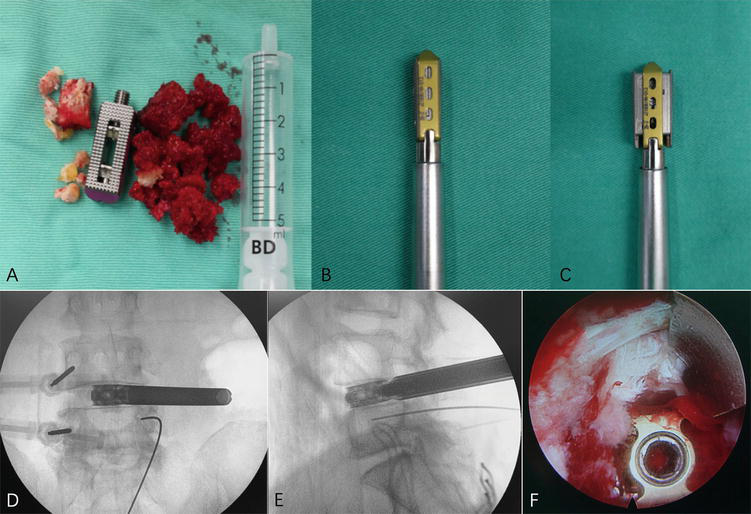
Figure 4.
Bone graft and interbody fusion cage implantation. (a–c) Height-adjustable cage, the autogenous bone, and allogenic bone are prepared for implantation. (d–e) The height-adjustable fusion cage is positioned at the center of the interbody space in the anteroposterior radiograph, and the leading edge reaches the position of the iliac crest. (f) The height-adjustable cage is confirmed in a satisfactory position under endoscopy. Nerves are not compressed by bone graft. Reproduced with permission from Yin et al. [
3.3 Unilateral biportal endoscopic transforaminal lumbar interbody fusion
UBE-TLIF combines the benefits of open and endoscopic surgery. UBE-TLIF is performed while the patient is prone under general or epidural anesthesia. All decompression and interbody fusion procedures are carried out utilizing a biportal endoscopic system. Briefly, the installation of watertight draperies and the establishment of portal sites under fluoroscopic guidance. Two ipsilateral skin incisions are made in the paramedian region, one centimeter above and one centimeter below the midpoint of the intervertebral space, as well as on the ipsilateral medial border of the pedicle. In the left-sided approach, the upper orifice serves as the endoscopic portal, whereas the lower hole is the functional portal. After making two small incisions in the epidermis and fascia, adequate portals are created using serial dilators. Using a specialized lamina dissector inserted through the working portal, the lamina is then dissected. During the procedure, an endoscopic irrigation system is utilized, and irrigation fluid is drained from the endoscopic portal to the working portal. If the irrigation flow is inadequate, a small endoscopic retractor can be utilized to enhance the flow, ensure adequate visibility, and reduce soft tissue edema. Using a radiofrequency coagulator, further osseous dissection and control of hemorrhage are performed. Using a tubular retractor and a microscope, the UBE-TLIF technique is comparable to the MIS-TLIF technique. Endoscopic burrs and Kerrison punches are used to conduct ipsilateral hemilaminectomy. After adequate ipsilateral decompression, the ligamentum flavum is removed by decompressing the contralateral sublaminar portion with sublaminar drilling. Using endoscopic burrs and osteotomes, a unilateral facetectomy is then conducted to harvest autograft bone. Complete exposure of the ipsilateral and contralateral nerve roots should be confirmed. Using pituitary forceps and reamers, the disc is radically removed after complete dorsal decompression. Under endoscopic observation, the cartilaginous endplate is removed completely using curettes. Under fluoroscopic guidance, bone fragments from the lamina and facet are impacted into the disc space, and an interbody fusion cage containing bone grafts and fusion material is inserted. The procedure concludes with the insertion of additional percutaneous pedicle screws and a drain catheter to prevent epidural hematoma.
4. Clinical effectiveness and safety
The main criteria for evaluating endoscopic TLIF remain clinical outcomes, complications, operating time, and surgical invasiveness. Compared to the conventional MIS-TLIF, a well-established endoscopic TLIF should present comparable clinical outcomes, complication rate, and operating time. In addition, it should result in less invasiveness, less estimated blood loss, and improved postoperative recovery.
4.1 Clinical outcomes
A large amount of research has confirmed that PE-TLIF, UBE-TLIF, and MIS-TLIF all have the capacity to achieve satisfactory clinical effects, with no significant differences in mid- to long-term outcomes. Several meta-analyses have reported that both PE-TLIF and UBE-TLIF are superior to MIS-TLIF in early relief of low back pain, especially within three months after surgery; however, there is no significant difference between the alleviation of leg discomfort and the enhancement of the Oswestry Disability Index [7, 8]. These results suggest that endoscopic TLIF could achieve adequate nerve decompression, with less ischemic damage to paraspinal muscles, ligaments, and posterior soft tissue.
4.2 Complications
In the early stages of application, the complication rate of endoscopic TLIF procedures was relatively high. In 2013, Jacquot et al. reported a complication rate of 36.8% in 57 cases of PE-TLIF surgery, mainly including nerve root injury and cage migration, and 13 cases (22.8%) required revision of surgery [9]. With advancements in endoscopic instruments and surgical techniques, the incidence of complications in endoscopic TLIF has decreased dramatically in recent years. A recent meta-analysis showed that the incidence of complications in PE-TLIF was 10.1%, which is close to that of MIS-TLIF (12.7%); the incidence of complications in UBE-TLIF (7.8%) was also similar to that of MIS-TLIF (7.1%) [8, 10]. The most common complications are dura tear, nerve injury, endplate injury, and screw misplacement. Most of the complications are mild in symptoms, which can be improved with conservative treatment.
4.2.1 Dural tear and nerve injury
Dural tear and nerve injuries are the most common complications of endoscopic TLIF surgery. In the literature, the incidence of these complications is 0.1-8% for PE-TLIF and 3-10% for UBE-TLIF, which is slightly higher than that in MIS-TLIF [11]. Endoscopic TLIF surgery may have a high risk of exiting nerve root irritation; however, the majority of these complications occurred during the initial phases of the learning curve and diminished as proficiency increased. Local anesthesia or general anesthesia with neuromonitoring may be an effective measure to reduce the risks of nerve injury.
4.2.2 Cage subsidence/migration
Cage subsidence or migration is a common complication in endoscopic TLIF surgery, with a slightly higher incidence than MIS-TLIF. Apart from osteoporosis, endplate injury is the culprit for cage subsidence. Intraoperatively, if the cage could not be implanted parallel to the endplate, the risk of endplate injury may increase. It is also important to note the bleeding from cancellous bone, which indicates injury to the bony endplate. Literature reports that in the early stages of PE-TLIF, the incidence of cage migration is 2 to 4%, while it is less common in UBE-TLIF [12, 13]. This may be due to the limited size of the instruments used, resulting in lower efficiency and insufficient handling of the intervertebral space.
4.2.3 Hematoma
According to previous studies, the incidence of postoperative hematoma in UBE-TLIF is higher than that of PE-TLIF, with an incidence of about 4% [14]. This may be due to the need to dissect more soft tissue and remove more bony structures in UBE-TLIF. The continuous fluid irrigation in the water-based endoscopic system may also impact the operation of hemostasis, causing an increased rate of postoperative hematoma. Active bleeding during the operation could be controlled by bipolar radiofrequency ablation, and hemostatic materials could be used to reduce the hemorrhage associated with epidural veins and bone surfaces.
4.2.4 Systemic complications
Research shows that the incidence of infection in endoscopic TLIF procedures is lower than that in MIS-TLIF, which may be attributed to the less invasiveness of endoscopic lumbar fusion surgery [15]. In terms of other systemic complications such as urinary retention, pneumonia, pulmonary embolism, and deep vein thrombosis, the endoscopic TLIF also presents a significantly lower incidence than MIS-TLIF. This may be related to enhanced recovery and early ambulation after endoscopic lumbar fusion surgery.
4.3 Operating time
Most literature reports a longer operating time in endoscopic TLIF procedures than that in MIS-TLIF [8]. The most time-consuming aspect of endoscopic lumbar fusion surgery is the handling of intervertebral space and endplate preparation. Although the improvement of endoscopic instruments has significantly increased the efficiency of these procedures, intraoperative evaluation of the endplate preparation status still requires a considerable amount of time. Unlike MIS-TLIF, which only requires palpation to assess the status of endplate preparation, endoscopic surgery needs additional visual observation or fluoroscopy to clearly ensure it.
4.4 Surgical invasiveness
MIS-TLIF necessitates extensive paraspinal muscle dissection and removal of the facet joint, partial lamina, and ligamentum flavum, whereas endoscopic TLIF aims to preserve these structures while ensuring adequate operative space, thereby significantly reducing surgical invasiveness. Both PE-TLIF and UBE-TLIF were shown by meta-analysis to substantially reduce intraoperative estimated blood loss compared to MIS-TLIF [8, 10]. In patients undergoing endoscopic TLIF, postoperative drainage, blood loss, and inflammatory indicators (e.g., C-reactive protein) were all reduced, and they also experienced a significantly shorter duration of ambulation and discharge from hospital. These effects of enhanced recovery after surgery are attributed to less invasiveness by endoscopic lumbar fusion surgery.
5. Learning curve
The learning curve for endoscopic TLIF is relatively long, and surgeons are required to have proficient endoscopic decompression experience before performing endoscopic lumbar fusion. In 2019, Kolcun et al. reported a case series of 100 consecutive patients undergoing PE-TLIF [16]. Complications occurred in four cases but three of which were developed in the initial stage of applying this procedure. Xu et al. suggested that at least 54 cases of endoscopic decompression were needed before performing UBE-TLIF [17]. Based on our experience, it is necessary to perform 20-30 endoscopic decompression procedures before the transition to more complex cases requiring lumbar interbody fusion.
6. Selection of fusion cages
In the early stages of endoscopic TLIF, the fusion process involved only interbody bone grafting, which resulted in a high incidence of postoperative bone graft absorption, displacement, and pseudarthrosis. As of now, fusion cages combined with autologous or synthetic bone implants are regarded as the gold standard. Based on the categories of materials used for fusion cages, they can be classified into metallic materials, polymer materials, and biomaterials.
The main types of metallic materials applied in endoscopic spine fusion cages are titanium alloys, which exhibit good mechanical properties and stability, making them suitable for the 3D printing of personalized and expandable fusion cages. However, metallic fusion cages have several disadvantages, such as a high elastic modulus, a high risk of subsidence, and metal artifacts, which can impact fusion status assessment. Polymer materials, such as polyetheretherketone (PEEK) and carbon fiber reinforced polymer (CFRP), are characterized by bone tissue-matched elastic modulus, low-stress shielding, and no artifacts. Additionally, fusion cages made of polymers can be designed into different shapes, such as bullet- or kidney-shaped, which are appropriate for endoscopic lumbar fusion surgery. Degradable polymers, such as polycarbonates, have excellent biocompatibility; however, the clinical application is limited by their high brittleness, long bone healing time, and adverse reactions caused by degradation products. Biomaterials, such as allogeneic bone, can shorten the duration of fusion but their low mechanical stiffness is not conducive to maintaining intervertebral height. Currently, expandable fusion cages made of titanium alloys or nonexpandable fusion cages made of polymer materials are the main alternative for endoscopic lumbar fusion surgery, but clinical trials with large sample sizes and long-term follow-up are still required.
Although there are various types of fusion cages, the safety of implantation procedures should be ensured. Fusion cages could be implanted through expandable channels and in the guidance of wires or tracks. Among the fusion cages that are commonly used, the size of titanium alloy expandable devices is relatively small, and they can be directly implanted through an endoscopic protective sheath tube. When passing through the Kambin triangle, expandable fusion cages have the least impact on nearby nerves, thus presenting high neuro-safety.
7. Fusion rate
According to clinical research, the fusion rate of endoscopic TLIF is comparable to that of MIS-TLIF. The fusion rate was 95.0% (360/379) and 94.9% (451/457) for patients undergoing PE-TLIF and MIS-TLIF, respectively [10]. The fusion rate between UBE-TLIF and MIS-TLIF is also comparable. However, some studies have indicated that the endoscopic TLIF would require more time to achieve fusion status than MIS-TLIF, with a fusion rate of 85.3% at 12 months for PE-TLIF and 92.3% for MIS-TLIF [2]. This discrepancy may be due to the continuous irrigation of bone surfaces with saline during endoscopic lumbar fusion procedures. Bone graft bed quality, bone graft material, fusion cage design, fusion status assessment criteria, and length of follow-up are all related to fusion success. Bone morphogenetic protein-2 has the potential to greatly increase the pace of fusion and decrease the time needed to achieve a fusion state.
8. Advanced techniques and future
Attributed to the development of imaging technologies and computer science, digital/intelligent surgery and implants would be the future of spine surgery. Robotic-assisted systems, mixed reality, and artificial intelligence (AI)–based surgical planning and implant design are expected to deeply integrate with endoscopic TLIF surgery. These techniques could further reduce surgical invasiveness, improve surgical efficiency, decrease the risks of neurological and vascular complications, and provide lower radiation to patients.
8.1 Robotic-assisted system
In the realm of spine surgery, a robot-assisted system is predominantly utilized for pedicle screw placement, presenting increased accuracy and diminished radiation exposure compared to traditional fluoroscopy [18]. Despite the similarities to O-arm navigations, a robot-assisted system could provide more precise physical guidance for surgeons to execute the surgical plan. Endoscopic lumbar fusion requires recurrent fluoroscopy for percutaneous pedicle screw insertion because the entry points of the pedicle screw are not visible. Therefore, the role of robotic-assisted surgery is more significant in endoscopic TLIF, and the safety and efficacy of the robot-assisted system in PE-TLIF have been well-validated by Chang et al. [19]. With the advent of an autonomous robotic system, decompression and facetectomy by a robot in endoscopic TLIF may be realized in the near future.
8.2 Mixed reality
Mixed reality (MR) technology is a combination of virtual reality and augmented reality, which anchors 3D hologram into the physical object and establishes an interactive feedback loop between the virtual and real world. In surgery, with the MR, a 3D anatomical model based on patients’ imaging data could be seamlessly integrated into the real surgical site, enabling surgeons to have a 3D vitalization and get real-time interaction. MR has been applied in various spine surgery, mainly for pedicle screw fixation [20]. Although it is similar to conventional navigation, MR is more adequate to facilitate synchronization between the image and the surgical field. Butler et al. reported the first case series of 164 patients with percutaneous pedicle screw fixation under the MR system [21]. In this study, only 3 minutes and 54 seconds were required from registration to placement for each screw, and high accuracy was achieved. Therefore, with the development of display and wearable devices, MR has the potential to be routinely used in endoscopic TLIF in the future.
8.3 Artificial intelligence–based surgical planning and implants design
A variety of AI automatic surgical planning and implant design systems for spine surgery have been reported. Caprara et al. employed an AI preoperative planning system to find the optimized pedicle screw trajectories by maximizing the CT-derived bone mechanical properties [22]. Recently, Ma et al. developed an innovative CT image-based AI technique for screw trajectory planning, including components of automatic vertebral detection, auto-simulation of pedicle screw placement, bone mineral density estimation, and screw pull-out simulations [23]. The optimized trajectories demonstrated significantly higher bone mineral density and pull-out force than the conventional trajectories, which would especially benefit patients with osteoporosis. Similarly, AI automatic design system for fusion cages was also developed. In addition to morphological matching, the fusion cages designed by this system have personalized biomechanical adaptability. Compared to titanium alloys or PEEK fusion cages, it possesses a more gradual stepwise elastic modulus, which matches the bone strength of patients. It is no doubt that the AI-based surgical planning and implant design system would provide more appropriate and personalized surgical planning for each patient, and it would have an important role in endoscopic TLIF surgery to further improve clinical outcomes.
9. Conclusion
Endoscopic TLIF requires proficiency in endoscopic decompression techniques as a foundation, coupled with a deep understanding of open fusion surgery or the MIS-TLIF technique. Surgeons should strictly select appropriate indications and choose suitable endoscopic lumbar fusion procedures based on their technique level and case characteristics, with an emphasis on surgical safety, effectiveness, and long-term clinical outcomes. Although the current limitations of endoscopic TLIF include a long learning curve, a lack of long-term interbody fusion outcomes, and the absence of standardized surgical procedures, the future prospects for endoscopic TLIF will be better with the improvement of endoscopic instruments, bone graft materials, imaging technologies, and AI system.
Conflict of interest
The authors declare no conflict of interest. This work was supported by Shoufa grant [2020-2-2038].
References
- 1.
Leu HF, Hauser RK, Schreiber A. Lumbar percutaneous endoscopic interbody fusion. Clinical Orthopaedics and Related Research. 1997; 337 :58-63 - 2.
Ao S, Zheng W, Wu J, Tang Y, Zhang C, Zhou Y, et al. Comparison of preliminary clinical outcomes between percutaneous endoscopic and minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative diseases in a tertiary hospital: Is percutaneous endoscopic procedure superior to MIS-TLIF? A prospective cohort study. International Journal of Surgery. 2020; 76 :136-143 - 3.
Wang JC, Cao Z, Li ZZ, Zhao HL, Hou SX. Full-endoscopic lumbar interbody fusion versus minimally invasive transforaminal lumbar interbody fusion with a tubular retractor system: A retrospective controlled study. World Neurosurgery. 2022; 165 :e457-ee68 - 4.
Kolcun JPG, Brusko GD, Wang MY. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: Technical innovations and outcomes. Annals of Translational Medicine. 2019; 7 (Suppl 5):S167 - 5.
Yin P, Ding Y, Zhou L, Xu C, Gao H, Pang D, et al. Innovative percutaneous endoscopic transforaminal lumbar interbody fusion of lumbar spinal stenosis with degenerative instability: A non-randomized clinical trial. Journal of Pain Research. 2021; 14 :3685-3693 - 6.
Yin P, Gao H, Zhou L, Pang D, Hai Y, Yang J. Enhanced recovery after an innovative percutaneous endoscopic transforaminal lumbar interbody fusion for the treatment of lumbar spinal stenosis: A prospective observational study. Pain Research & Management. 2021; 2021 :7921662 - 7.
Guo H, Song Y, Weng R, Tian H, Yuan J, Li Y. Comparison of clinical outcomes and complications between endoscopic and minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative diseases: A systematic review and meta-analysis. Global Spine Journal. 2022:21925682221142545 - 8.
Yang H, Cheng F, Hai Y, Liu Y, Pan A. Unilateral biportal endoscopic lumbar interbody fusion enhanced the recovery of patients with the lumbar degenerative disease compared with the conventional posterior procedures: A systematic review and meta-analysis. Frontiers in Neurology. 2022; 13 :1089981 - 9.
Jacquot F, Gastambide D. Percutaneous endoscopic transforaminal lumbar interbody fusion: Is it worth it? International Orthopaedics. 2013; 37 (8):1507-1510 - 10.
Zhu L, Cai T, Shan Y, Zhang W, Zhang L, Feng X. Comparison of clinical outcomes and complications between percutaneous endoscopic and minimally invasive transforaminal lumbar interbody fusion for degenerative lumbar disease: A systematic review and meta-analysis. Pain Physician. 2021; 24 (6):441-452 - 11.
Ahn Y, Youn MS, Heo DH. Endoscopic transforaminal lumbar interbody fusion: A comprehensive review. Expert Review of Medical Devices. 2019; 16 (5):373-380 - 12.
Nagahama K, Ito M, Abe Y, Murota E, Hiratsuka S, Takahata M. Early clinical results of percutaneous endoscopic Transforaminal lumbar interbody fusion: A new modified technique for treating degenerative lumbar spondylolisthesis. Spine Surgery and Related Research. 2019; 3 (4):327-334 - 13.
Butler AJ, Brusko GD, Wang MY. Awake endoscopic Transforaminal lumbar interbody fusion: A technical note. HSS Journal. 2020; 16 (2):200-204 - 14.
Youn MS, Shin JK, Goh TS, Lee JS. Full endoscopic lumbar interbody fusion (FELIF): Technical note. European Spine Journal. 2018; 27 (8):1949-1955 - 15.
Zhao XB, Ma HJ, Geng B, Zhou HG, Xia YY. Early clinical evaluation of percutaneous full-endoscopic transforaminal lumbar interbody fusion with pedicle screw insertion for treating degenerative lumbar spinal stenosis. Orthopaedic Surgery. 2021; 13 (1):328-337 - 16.
Kolcun JPG, Brusko GD, Basil GW, Epstein R, Wang MY. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: Operative and clinical outcomes in 100 consecutive patients with a minimum 1-year follow-up. Neurosurgical Focus. 2019; 46 (4):E14 - 17.
Xu J, Wang D, Liu J, Zhu C, Bao J, Gao W, et al. Learning curve and complications of unilateral Biportal endoscopy: Cumulative sum and risk-adjusted cumulative sum analysis. Neurospine. 2022; 19 (3):792-804 - 18.
Li HM, Zhang RJ, Shen CL. Accuracy of pedicle screw placement and clinical outcomes of robot-assisted technique versus conventional freehand technique in spine surgery from nine randomized controlled trials: A meta-analysis. Spine (Phila Pa 1976). 2020; 45 (2):E111-E1e9 - 19.
Chang M, Wang L, Yuan S, Tian Y, Zhao Y, Liu X. Percutaneous endoscopic robot-assisted transforaminal lumbar interbody fusion (PE RA-TLIF) for lumbar spondylolisthesis: A technical note and two years clinical results. Pain Physician. 2022; 25 (1):E73-e86 - 20.
Lu L, Wang H, Liu P, Liu R, Zhang J, Xie Y, et al. Applications of mixed reality Technology in Orthopedics Surgery: A pilot study. Frontiers in Bioengineering and Biotechnology. 2022; 10 :740507 - 21.
Butler AJ, Colman MW, Lynch J, Phillips FM. Augmented reality in minimally invasive spine surgery: Early efficiency and complications of percutaneous pedicle screw instrumentation. The Spine Journal. 2023; 23 (1):27-33 - 22.
Caprara S, Fasser MR, Spirig JM, Widmer J, Snedeker JG, Farshad M, et al. Bone density optimized pedicle screw instrumentation improves screw pull-out force in lumbar vertebrae. Computer Methods in Biomechanics and Biomedical Engineering. 2022; 25 (4):464-474 - 23.
Ma C, Zou D, Qi H, Li C, Zhang C, Yang K, et al. A novel surgical planning system using an AI model to optimize planning of pedicle screw trajectories with highest bone mineral density and strongest pull-out force. Neurosurgical Focus. 2022; 52 (4):E10