Summary of inhibitory effects of the statins on Kv1.3 channels.
Abstract
Statins are organic compounds, which are applied in medicine, basically to reduce blood cholesterol level. Studies performed during past years provided evidence that statins may also be applied in the therapy of some types of cancer, such as colorectal cancer, breast cancer, or leukemia. Anticancer activity of statins may be due to the inhibition of voltage-gated potassium channels Kv1.3. Inhibition of these channels may exert antiproliferative and pro-apoptotic effects on Kv1,3 channel-expressing cancer cells. This may lead to a selective apoptosis of the cancer cells while sparing the normal ones. This chapter focuses on the inhibitory effects of statins on Kv1.3 channels and on the antiproliferative and pro-apoptotic effects of these compounds on Kv1.3 channel-expressing cancer cells. It is shown that the statins lovastatin, mevastatin, pravastatin, and simvastatin are effective inhibitors of the channels expressed in cancer cell line Jurkat T. The channel inhibition may be related to the anticancer activities of these compounds. Moreover, pro-apoptotic activity of the compounds is significantly augmented upon co-application of the statins with flavonoids and xanthohumol. This may be related to an additive or synergistic inhibition of Kv1.3 channels in these cells by the compounds applied in combination.
Keywords
- statins
- flavonoids
- Kv1.3 channel
- cancer cell proliferation
- cancer cell apoptosis
1. Introduction
Statins are organic compounds, which can be obtained from plants, such as
Statins are known as inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which plays a crucial role in the biosynthesis of cholesterol [1]. Therefore, statins may widely be applied in the treatment of hypercholesterolemia and atherosclerosis. According to the recently formulated hypothesis, statins may also be applied in the treatment of severe COVID-19 cases. This may due to the inhibition of cholesterol biosynthesis, which leads to a reduction of cholesterol content and prevention of SARS-CoV-2 virus’s entry into host cells [2].
It is known that statins may exert pleiotropic effects, which are far beyond their ability for inhibition of biosynthesis of cholesterol. It is known that statins may also exert anticancer activities. It was shown that statins may exert antiproliferative and proapoptotic effects on many cell lines of many different types of cancers [1]. Among cancer cell lines, which are targets for statins, are breast cancer cells, colon carcinoma, glioblastoma, leukemia, melanoma, myeloma, pancreatic cancer, prostate cancer, and thyroid cancer [1]. The statins that are active as anticancer agents are: lovastatin, cerivastatin, fluvastatin, simvastatin, pravastatin, and atorvastatin [1].
Recently published data has provided evidence that the statins simvastatin and mevastatin exert antiproliferative and proapoptotic effects on human colorectal adenocarcinoma cell line LoVo and its doxorubicin-resistant subline LoVo/DX [3]. Importantly, application of the statins re-sensitized the LoVo/DX cells to doxorubicin treatment. Interestingly, the anticancer activities of the statins were significantly augmented when the statins were co-applied with flavonoids 6-hydroxyflavone, 7-hydroxyflavone, and baicalein [3]. It was shown that the statins and the flavonoids may act synergistically when co-applied with each other. The most significant synergism was observed upon the co-application of simvastatin with baicalein on LoVo/DX cells [3].
Flavonoids are a wide group of plant-derived compounds, which exert pleiotropic effect on many molecular targets. Among them are voltage-gated potassium channels Kv1.3, which are inhibited by some flavonoids, such as genistein, acacetin, chrysin, natural (prenyl), and synthetic (methoxy) derivatives of naringenin [4]. Similar inhibitory effects on the channels are exerted by some chalcones, such as xanthohumol [4] or isobavachalcone (Teisseyre – unpublished results).
Voltage-gated potassium channels (Kv) are integral membrane proteins, which are selectively permeable for potassium ions and are activated upon a change of the cell membrane voltage. Activation of these channels enables transportation of potassium ions across the cell membrane down the electrochemical gradient. The channels are known as “delayed rectifier” Kv channels, which activate upon membrane depolarization and undergo a slow and complex C-type inactivation [4, 5]. Activation of Kv1.3 channels in the plasma membrane provides an efflux of potassium ions out of the cell and stabilization of the resting membrane potential [5]. Kv1.3 channels are mammalian
Several studies have demonstrated altered expression of Kv1.3 in some cancer types when comparing with normal tissue [4, 13, 14, 15, 16]. However, no general pattern of these changes is known at present. The changes depend on the type and the stage of the disease. Cancer tissues may upregulate or downregulate the channels. An increased expression of Kv1.3 channels was observed in the case of breast, colon, smooth muscle (leiomyosarcoma), skeletal muscle (alveolar rabdomyosarcoma), and lymph node cancers and in mature neoplastic B cells in chronic lymphocytic leukemia (B-CLL) [4]. On the other hand, a markedly reduced expression of Kv1.3 channels was detected in a case of breast adenocarcinoma, and there was an inverse correlation between the channel expression and the disease’s grade [4]. A significantly reduced expression of the channels was also observed in kidney, bladder, pancreas, lung, brain (astrocytoma, oligodendroglioma, and glioblastoma), stomach, and prostate cancers [4].
Importantly, many different cancer cells lines, which are affected by anticancer activities of statins, express Kv1.3 channels both in the plasma membrane and in mitochondria [1, 4]. Among them are breast cancer cell lines: MCF-7 and MDA-MB-231, colon carcinoma SW-480 and LoVo cells, glioblastoma U87 cell line, leukemic Jurkat T and CEM cells, acute myeloid leukemia (OCI-AML-3) cell line, promyeloytic leukemia HL-60 cell line, pancreatic cancer PANC-1 cell line, and prostate cancer LNCaP cell line [1, 4]. It is known that membrane-permeant small-molecule organic inhibitors of Kv1.3 channels may be able to simultaneously inhibit proliferation of Kv1.3 channel-expressing cancer cells (by the inhibition of plasma membrane Kv1.3 channels) and to induce apoptosis of these cells (by the inhibition of the mito Kv1.3 channels) [4]. The apoptosis of Kv1.3 channel-expressing cancer cells occurred by an activation of the intracellular (mitochondrial) pathway of this process [4]. Importantly, the apoptosis occurred only in the cancer but not in normal cells [4, 17, 18]. Therefore, these inhibitors may potentially be applied in the treatment of some cancer diseases such as, melanoma, pancreatic ductal adenocarcinoma (PDAC), multiple myeloma, and B-type chronic lymphocytic leukemia (B-CLL) [4, 17, 18, 19, 20].
In contrast to flavonoids, inhibitory effects of statins on Kv1.3 channels in cancer cells remain unknown. Therefore, in order to elucidate the role of inhibition of these channels in antiproliferative and pro-apoptotic activities of statins on cancer cells, the influence of statins on the activity of Kv1.3 channels in cancer cells needs to be elucidated. Then, the relationship between the putative channel inhibition and anticancer activities of statins must be studied in detail. Since it is known that these activities are augmented upon co-application with flavonoids, effects of co-application of the statins simvastatin and mevastatin and flavonoids on the activity of Kv1.3 channels and the viability of Kv1.3 channel-expressing cancer cells should be elucidated.
2. Influence of statin application on the activity of Kv1.3 channels and viability of Kv1.3 channel-expressing cancer cells
2.1 Influence of statins on the activity of Kv1.3 channels in normal and cancer cells
The first report about the inhibitory effect of statins on Kv1.3 channels appeared in the literature only in 2014 [21]. The authors were looking for immunomodulatory agents that can be applied as anti-inflammatory drugs. They applied the “whole-cell” patch-clamp technique [22] to study the influence of the statins pravastatin, lovastatin, and simvastatin on the activity of Kv1.3 channels in mice thymocytes. They observed that application of 1 mM of pravastatin significantly reduced the end-of-the-pulse whole-cell Kv1.3 currents, elicited by a sequence of depolarizing voltage steps, probably due to acceleration of the current inactivation rate. This effect was irreversible, since the inactivation rate did not recover to the control value after wash-out of the drug [21].
The authors observed that other tested statins lovastatin and simvastatin exerted much more significant inhibitory effect on the currents at much lower concentrations. Application of both statins at the concentration of 10 μM significantly reduced both the peak and the end-of-the-pulse Kv1.3 currents elicited by a sequence of depolarizing voltage steps. The reduction of the end-of-the-pulse current was more significant than the reduction of the peak current, probably due to acceleration of current inactivation rate upon application of the statins. The inhibitory effect exerted by lovastatin was reversible, because the currents recovered to the control value after wash-out of the drug, but the one exerted by simvastatin was irreversible [21]. Measurements of membrane capacitance showed that application of lovastatin and simvastatin, but not pravastatin, led to a significant decrease of the membrane capacitance. This decrease was abolished after the wash-out of lovastatin, but it remained after the wash-out of simvastatin [21]. The decrease of membrane capacitance was probably due to an increase of membrane thickness upon application of the statins. The increase of membrane thickness may be a consequence of interactions between the statin molecules and the lipid bilayer, leading to perturbations of the bilayer structure. These perturbations were reversible upon application of lovastatin, but irreversible in the case of application of simvastatin. Perturbations of the lipid bilayer structure indirectly reduced the channel activity [21].
A more detailed study on the influence of application of lovastatin on the activity of Kv1.3 channels was performed by Zhao and co-workers [23]. The authors were motivated by a need for the search of immunomodulatory agents to be applied in the treatment of immune-related disorders, especially in T-lymphocyte-mediated autoimmune diseases. The influence of lovastatin on the activity of Kv1.3 channels was studied on the channels expressed both in normal cells – human T lymphocytes isolated from peripheral blood of healthy donors – and in Kv1.3 channel-expressing human lymphoblastic cancer cell line Jurkat, applying the “whole-cell” patch-clamp technique [23]. It was shown that lovastatin inhibited both the peak and the end-of-the-pulse Kv1.3 currents, elicited by a sequence of depolarizing voltage steps, in the concentration-dependent manner. The inhibition of the end-of-the-peak current was more potent than the inhibition of the peak current. This is probably due to a significant acceleration of the current inactivation rate upon application of lovastatin. The inhibitory effect was reversible at all the concentrations between 1 and 100 μM [23]. The half-blocking concentration values (IC50) were equal to 39.81 ± 5.11 μM and 6.92 ± 0.95 μM for the peak and end-of-the-pulse current, respectively [23]. Importantly, the inhibitory effects exerted by lovastatin on the channels expressed in normal and cancer cells were indistinguishable from each other [23]. Therefore, the inhibitory effect of lovastatin on Kv1.3 channels may exert not only the expected immunomodulatory effect but also antiproliferative effect on Kv1.3 channel-expressing cancer cells.
Importantly, it was shown that the inhibitory effect of lovastatin on the channels was significantly diminished when lovastatin was co-applied with internal tetraethylamine (TEA) or external verapamil [23]. Both of these drugs are inhibitors of Kv1.3 channels [5]. Moreover, the inhibition was significantly weaker in the case of mutant channels, where valine in the position 417 was replaced by alanine (V417A) [23]. This suggests that the channel inhibition is not only a simple consequence of perturbations of the structure of lipid bilayer, but it is also, at least partially, due to the binding of lovastatin molecules to a binding site on the channel protein. This binding site probably overlaps the binding site for internal TEA and external verapamil. Therefore, a co-application of the statin with these drugs leads to a reduction of the lovastatin-induced channel inhibition, due to a competition for the binding site. The inhibition of Kv1.3 channels by lovastatin probably occurs via an “open channel block”- like mechanism [23].
The inhibition of Kv1.3 channels in cancer cells may also occur in the case of application of other statins, which are inhibitors of the channels in normal cells, such as pravastatin and simvastatin [21]. Another possible candidate to be an inhibitor of Kv1.3 channels in cancer cells is mevastatin. This compound is structurally closely related both to lovastatin and to simvastatin, and it shares anticancer activities of simvastatin on Kv1.3 channel-expressing cell line LoVo [3].
A detailed study on the influence of simvastatin, mevastatin, and pravastatin on the activity of Kv1.3 channels in cancer cells was performed in the past years by Teisseyre and co-workers [24]. Since Kv1.3 channels are endogenously and abundantly expressed in human leukemic T cell line Jurkat, these cells were used as a model system of Kv1.3 channel-expressing cancer cells. The study was performed applying the “whole-cell” patch-clamp technique [24].
The authors showed that the application of each one of the statins caused a significant decrease of the peak whole-cell Kv1.3 current recorded upon the application of depolarizing “voltage ramps” (Figure 1A, upper panel, [24]). In an agreement with the results obtained earlier by Kazama and co-workers [21], the least potent inhibition of the currents occurred upon the application of pravastatin. Application of this statin at the concentrations up to 50 μM caused a concentration-dependent reduction of the current amplitude to about 0.74 of the initial value. This was accompanied by a remarkable but statistically insignificant acceleration of the current inactivation rate [24]. In contrast to what was observed by Kazama and co-workers [21], the inhibitory effect of pravastatin on the channels was fully reversible, probably because the applied concentrations were much lower (50 μM was a maximum) [24].
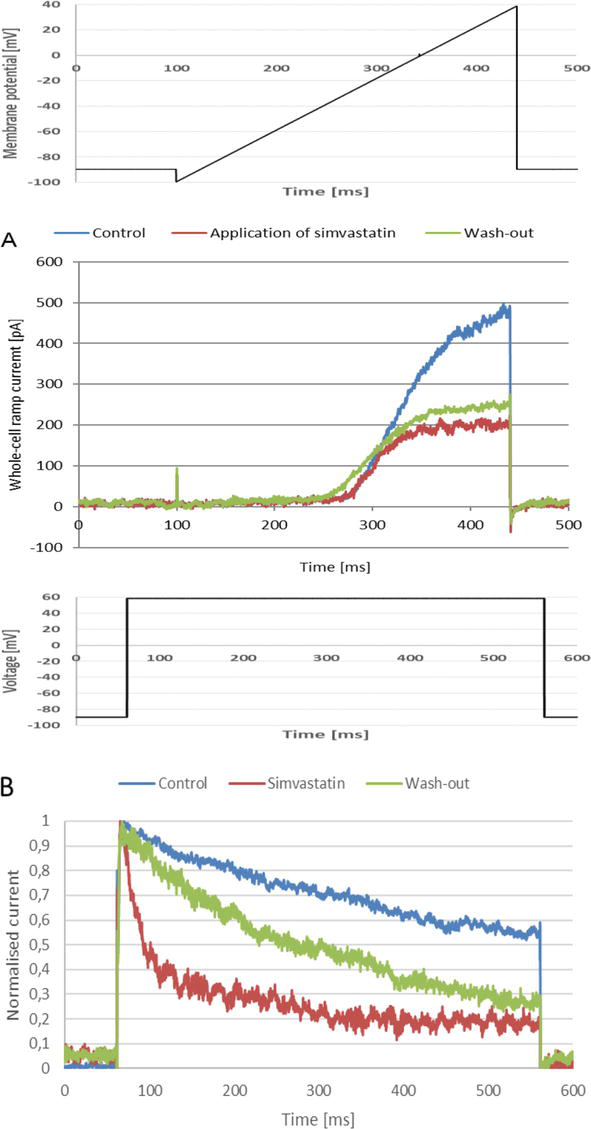
Figure 1.
(A) Whole-cell Kv1.3 ramp currents (lower panel) recorded in a Jurkat T cell upon an application of the depolarizing “voltage ramp” (upper panel), (B) normalized whole-cell Kv1.3 currents (lower panel) recorded on the same cell upon an application of a depolarizing voltage step (upper panel). The concentration of simvastatin was equal to 30 μM.
A much more significant inhibition of the channel activity occurred upon the application of both simvastatin and mevastatin [24]. Application of simvastatin caused a concentration-dependent reduction of the peak “ramp current” to about 0.28 of the control value (Figure 1A, lower panel). The value of the IC50 parameter was equal to 4.85 ± 0.011 μM [24]. The current did not recover after “wash-out” of the statin (Figure 1A, lower panel). This is in accordance with what was observed by Kazama and co-workers, who showed that the inhibitory effect of simvastatin was partially irreversible [21].
The reduction of the current amplitude was accompanied by a significant acceleration of the current inactivation (Figure 1B, lower panel), which was revealed by a large reduction of the value of inactivation time constant, calculated for the currents recorded upon the application of depolarizing voltage steps (Figure 1B, upper panel) [24]. On the other hand, no significant change of the current activation rate was observed (Figure 1B, lower panel, [24]).
The inactivation phase of the currents did not recover completely after the wash-out of the drug (Figure 1B, lower panel) [24]. This indicates that the acceleration of inactivation was partially irreversible. Such a partial irreversibility was observed at all the concentrations applied [24]. This is in accordance with what was observed earlier by Kazama and co-workers [21].
Application of mevastatin caused a concentration-dependent reduction of the peak “ramp current” to about 0.32 of the control value at the concentration of 30 μM (Figure 2A) [24].
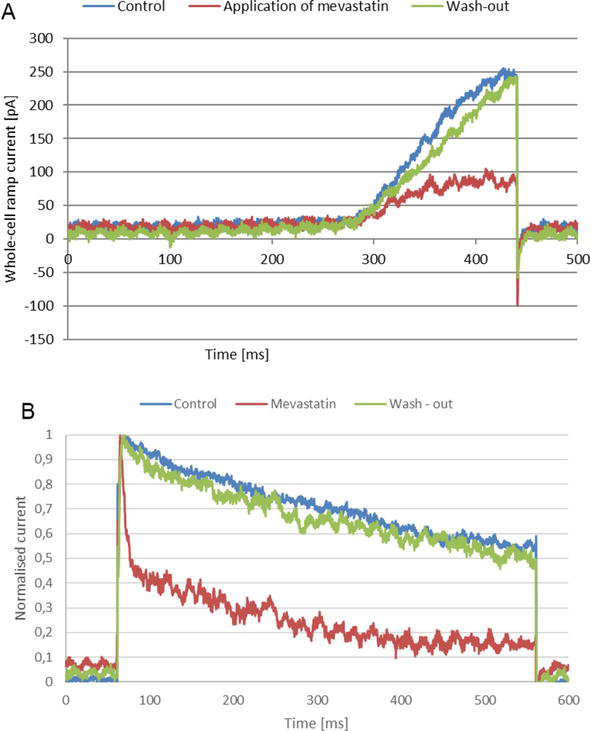
Figure 2.
(A) Whole-cell Kv1.3 ramp currents, (B) normalized whole-cell Kv1.3 currents recorded on a Jurkat T cell applying experimental protocols same as in
The value of the IC50 parameter was equal to 6.04 ± 0.4 μM [24]. Thus, the inhibitory effect exerted on the channels by mevastatin was comparable to the one exerted by simvastatin. However, in contrast to what was observed for simvastatin, the inhibitory effect of mevastatin was fully reversible at all applied concentrations (Figure 2A) [24].
The reduction of the current amplitude was accompanied by a significant acceleration of the current inactivation, which was revealed by a large reduction of the value of inactivation time constant, calculated for the currents recorded upon the application of depolarizing voltage steps (Figure 2B) [24]. On the other hand, no significant change of the current activation rate was observed (Figure 2B). However, in contrast to what was observed for simvastatin, the inactivation phase of the currents recovered completely after the wash-out of mevastatin (Figure 2B) [24]. Thus, the acceleration of inactivation was fully reversible, and such a reversibility was observed at all applied concentrations [24].
The inhibitory effects exerted on Kv1.3 channels in Jurkat T cells by simvastatin and mevastatin resemble the effect exerted by lovastatin [23]. Also, in the case of application of lovastatin, the channel inhibition is accompanied by a significant acceleration of the whole-cell Kv1.3 currents, without a significant change of the activation rate [23]. The mechanism of Kv1.3 channel inhibition by the statins is probably complex and includes both specific interactions of the statin molecules with a binding site on the channel protein in an “open channel block”–like mechanism and nonspecific interactions with the lipid bilayer, leading to perturbations in the bilayer’s structure, which, in turn, affect the channel activity. Both specific and nonspecific interactions finally lead to a stabilization of the channel proteins in an inactivated (nonconducting) state [24]. Since pravastatin is much less lipophilic than the others, the mechanism of its inhibitory effect is probably different, and it may be related to the interactions of the statin molecules with the external vestibule of the channel [24].
Finally, Wang and co-workers showed that application of simvastatin significantly inhibited mRNA and protein expression of Kv1.3 channels in human monocytic leukemia THP-1 cells [25]. Electrophysiological recordings performed applying the “whole-cell” patch-clamp technique showed that application of simvastatin in the range of 1–100 μM reduced the peak whole-cell Kv1.3 current recorded upon application of a sequence of depolarizing voltage steps [25]. The value of the IC50 parameter was equal to 8.75 ± 1.25 μM [25]. This value was significantly higher than the value calculated by Teisseyre and co-workers [24]. Moreover, in contrast to what was observed by other authors [21, 24], application of simvastatin in monocytes did not accelerate the inactivation of the recorded “whole-cell” potassium currents [25]. This is probably due to the fact that the recorded currents are apparently and significantly contaminated by slowly activating and non-inactivating voltage-gated potassium currents, which are resistant both to simvastatin and to a specific Kv1.3 channel inhibitor, margatoxin (MgTX) [25]. These currents, which may be due to an activation of an unknown type of voltage-gated potassium channel, may mask the effect of simvastatin on the inactivation kinetics of Kv1.3 currents.
The influence of the statins pravastatin, lovastatin, simvastatin, and mevastatin on the activity of Kv1.3 channels is summarized in Table 1.
| Name of the statin | Inhibitory effect | Maximal inhibition | References |
|---|---|---|---|
| Pravastatin | Dose-dependent reduction of the “whole-cell” current combined with acceleration of inactivation. Partially irreversible at the concentration of 1 mM | Ca. 36% at the concentration of 1 mM | [21, 24] |
| Lovastatin | Dose-dependent reduction of the “whole-cell” current combined with acceleration of inactivation. Reversible | Ca. 90% at the concentration of 100 μM | [21, 23] |
| Simvastatin | Dose-dependent reduction of the “whole-cell” current combined with acceleration of inactivation. Partially irreversible | Ca. 80% at the concentration of 100 μM | [21, 24, 25] |
| Mevastatin | Dose-dependent reduction of the “whole-cell” current combined with acceleration of inactivation. Reversible | Ca. 68% at the concentration of 30 μM | [24] |
Table 1.
2.2 Anti-proliferative and pro-apoptotic activities of the statins on Kv1.3 channel-expressing cancer cells
The mechanism of antiproliferative effect of statins on Kv1.3 channel-expressing cancer cells was studied in detail in the case of application of lovastatin on human leukemic T-cell line Jurkat, which can be considered as a model system of Kv1.3 channel-expressing cancer cells [23]. It was shown that the application of lovastatin caused a concentration-dependent decrease of influx of calcium ions through calcium release-activated channels (CRAC) [23]. This, in turn, caused a concentration-dependent down-regulation of calcium-related transcription factors: NFAT1 (nuclear factor of activated T cells) and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) [23]. Finally, the application of lovastatin caused a concentration-dependent inhibition of production of T-cell growth factor Interleukin-2 (IL-2) and cell proliferation [23]. The antiproliferative effect of lovastatin was partially but not completely abrogated when Kv1.3 channels were knocked down by applying the Kv1.3 channel-specific Kv1.3 small-interfering RNA (Kv1.3-siRNA) [23]. This indicates that the antiproliferative effect of lovastatin on Kv1.3 channel-expressing cancer cells is complex, and it includes both Kv1.3 channel-dependent and channel-independent pathways [23].
The Kv1.3 channel-dependent antiproliferative effect of lovastatin on Jurkat T cells is typical for Kv1.3 channel inhibitor-mediated inhibition of proliferation of normal and cancer cells expressing these channels. According to the theoretical “membrane potential model,” inhibition of Kv1.3 channels leads to depolarization of the cell membrane and reduction of the electrochemical “driving force” for entry of calcium ions through the calcium CRAC channels (Figure 3) [4]. Reduction of calcium ion influx inhibits all the downstream calcium-dependent processes, including activation of transcription factors, Interleukin-2 production, and, finally, the cell proliferation [4]. In such a case, the cell cycle is halted at the checkpoint between the G1 and the S phase [4, 5, 8, 9, 10].
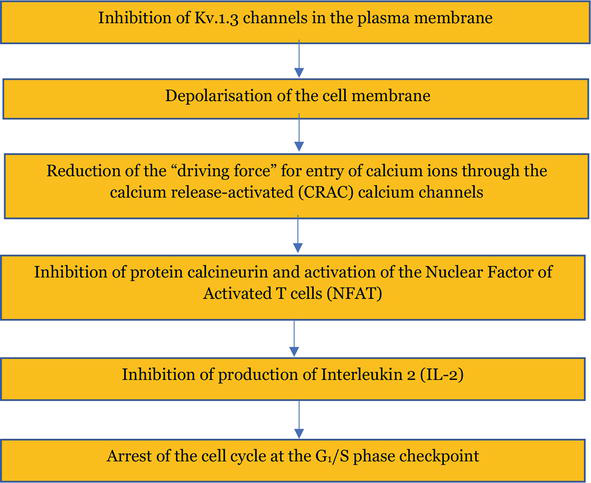
Figure 3.
Scheme of events upon inhibition of proliferation of Kv1.3 channel-expressing cells by the channel inhibitors according to the “membrane potential model.”
Lovastatin exerts antiproliferative effect on many other cancer cell lines expressing Kv1.3 channels. Among them are breast cancer MCF-7 and MDA-MB-231 cell lines; colon carcinoma HCT116, SW480, and LoVo cells; glioblastoma U87 cell line; leukemic CEM and OCI-AML 3 cell lines; promyelotic leukemia HL60 cell line; pancreatic cancer PANC-1 cells; and prostate cancer LNCaP cells [1]. Interestingly, the antiproliferative effect of lovastatin on Kv1.3 channel-expressing cancer cells is shared by other statins, which are inhibitors of Kv1.3 channels in these cells. Pravastatin inhibits proliferation of leukemic Jurkat T and CEM cells, whereas simvastatin, in addition to the leukemic cell lines mentioned above, also inhibits proliferation of breast cancer MCF-7 and MDA-MB-231 cell lines [1]. Moreover, simvastatin and mevastatin inhibit proliferation of colon carcinoma LoVo cells and their doxorubicin-resistant subline LoVo/DX [3]. Whether these antiproliferative effects are related to inhibition of Kv1.3 channels remains to be elucidated.
It is known that lipophilic small-molecule organic inhibitors of Kv1.3 channels may induce apoptosis of Kv1.3 channel-expressing cancer cells by an inhibition of mito Kv1.3 channels [4]. The apoptosis occurs by activation of its intracellular (mitochondrial) pathway. Inhibitors of mito Kv1.3 channels mimic the action of the pro-apoptotic protein Bax. The inhibition of mito Kv1.3 channels facilitates the production of Reactive Oxygen Species (ROS) by mitochondria. This, in turn, promotes opening of mitochondrial Permeability Transition Pores (PTP), loss of mitochondrial membrane potential (MMP), release of mitochondrial cytochrome c, and activation of pro-apoptotic enzymes, caspase-9 and caspase-3 (Figure 4) [4].
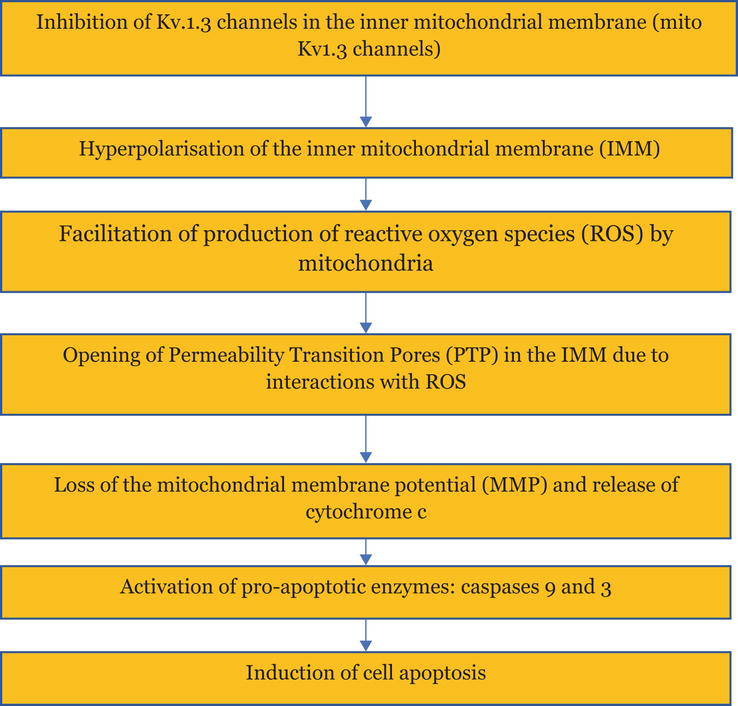
Figure 4.
Scheme of events upon induction of apoptosis of Kv1.3 channel-expressing cancer cells by the inhibitors of mito Kv1.3 channels.
Importantly, as it was mentioned previously, induction of cancer cell apoptosis by inhibitors of mito Kv1.3 channels may occur only in Kv1.3 channel-expressing cancer cells, whereas normal cells expressing these channels are saved [4]. This is because of a combination of an elevated expression of Kv1.3 channels in some cancer cells and elevated basal ROS production in these cells [4]. Such a combination does not occur in normal cells, even in those that overexpress Kv1.3 channels [4].
It was shown that the application of simvastatin and mevastatin induced apoptosis of Kv1.3 channel-expressing cancer cell line LoVo and its doxorubicin-resistant subline LoVo/Dx [3]. Application of the statins induced activity of caspase-3 and fragmentation of DNA in the above-mentioned cell lines [3]. Results published recently by Teisseyre and co-workers [26] showed that the application of both simvastatin and mevastatin induced apoptosis of Jurkat T cells. The pro-apoptotic activity was stronger in case of mevastatin than upon the application of simvastatin. The concentration required to reduce viability of the cells to 50% of the control value (IC50) was equal to 22.5 μM in case of mevastatin application. In case of application of simvastatin, the viability was reduced to about 60% even upon application of 40 μM of the statin. The increase of activity of caspase-3 and elevation of cleaved caspse-3 in these cells upon application of the statins were accompanied by a loss of the mitochondrial membrane potential (MMP) [26]. This may indicate that the statin-induced apoptosis of Jurkat T cells is a result of the activation of the mitochondrial pathway of this process. The apoptosis may be triggered by inhibition of mito Kv1.3 channels by the statins (Figure 4).
The pro-apoptotic activity of simvastatin and mevastatin on Kv1.3 channel-expressing cancer cells is shared by another statin, which is an inhibitor of Kv1.3 channels in cancer cells, lovastatin [1]. It was shown that the application of lovastatin induced apoptosis of Kv1.3 channel-expressing colon carcinoma HCT116, SW480, and LoVo cell lines; human leukemic Jurkat T and CEM cells; promyelocytic leukemia HL60 cells; and prostate cancer LNCaP cell line [1]. Moreover, the application of pravastatin induced apoptosis of human leukemic Jurkat T and CEM cells [1].
A short list of Kv1.3 channel-expressing cancer cells, on which statin inhibitors of the channels exert antiproliferative and anti-apoptotic effects, is given in Table 2.
| Name of the statin | Antiproliferative and pro-apoptotic effects on Kv1.3 channel-expressing cancer cells | References |
|---|---|---|
| Pravastatin | Human leukemic Jurkat T and CEM | [1] |
| Lovastatin | Human leukemic Jurkat T, CEM, and OCI-AML 3; breast cancer MCF-7 and MDA-MB-231; colon carcinoma HCT116, SW480, and LoVo; glioblastoma U87; promyelotic leukemia HL60; pancreatic cancer PANC-1; prostate cancer LNCaP | [1, 23] |
| Simvastatin | Human leukemic Jurkat T, CEM; breast cancer MCF-7 and MDA-MB-231; colon carcinoma LoVo and LoVo/DX | [1, 3, 26] |
| Mevastatin | Human leukemic Jurkat T; colon carcinoma LoVo and LoVo/DX | [3, 26] |
Table 2.
List of Kv1.3 channel-expressing cancer cells, on which the statins exert antiproliferative and pro-apoptotic effects.
Whether these pro-apoptotic activities are related to inhibition of mito Kv1.3 channels remains to be elucidated.
2.3 Influence of co-application of statins and flavonoids on the activity of Kv1.3 channels and viability of Kv1.3 channel-expressing cancer cells
Effects of co-application of the statins simvastatin and mevastatin and the flavonoids 8-prenylnaringenin (8-PR), 6-prenylnaringenin (6-PR), acacetin (Ac), chrysin (Ch), and a chalcone xanthohumol (X) on the activity of Kv1.3 channels and the viability of Kv1.3 channel-expressing human leukemic T cell line Jurkat were studied in detail recently [26]. Each of these compounds is an effective inhibitor of Kv1.3 channels in Jurkat T cells [4, 24]. Moreover, the application of 6-PR, Ac, and Ch significantly reduced the viability of Jurkat T cells [27]. It is shown that co-application of mevastatin with all the flavonoids and xanthohumol significantly augmented the inhibitory effect of mevastatin on Kv1.3 channels in Jurkat T cells [26]. Moreover, a significant augmentation of the inhibitory effect exerted on the channels by simvastatin was observed upon a co-application of this statin with 8-PR, 6-PR, and Ch [26].
In most cases, the inhibitory effect exerted on Kv1.3 channels upon co-application of the statins with the flavonoids may be considered as additive [26]. In such a case, the relative peak current recorded upon co-application of the statins with the flavonoids is equal to the product of multiplication of the relative currents recorded upon application of each compound alone. Table 3 shows comparisons of relative peak currents recorded upon co-application of the statins with the flavonoids with theoretical values calculated applying the additive inhibition model [26].
| Simvastatin co-applied with: | 8-PR | 6-PR | X | Ac | Ch |
|---|---|---|---|---|---|
| Theoretical values | 0.195 | 0.395 | 0.36 | 0.32 | 0.285 |
| Experimental values | 0.12 | 0.26 | 0.50 | 0.53 | 0.25 |
| Inhibitory effect upon co-application | Synergistic | Synergistic | Not additive | Not additive | Additive |
| Mevastatin co-applied with: | 8-PR | 6-PR | X | Ac | Ch |
| Theoretical values | 0.16 | 0.32 | 0.31 | 0.26 | 0.23 |
| Experimental values | 0.09 | 0.29 | 0.30 | 0.30 | 0.26 |
| Inhibitory effect upon co-application | Synergistic | Additive | Additive | Additive | Additive |
Table 3.
Comparison of theoretical and experimental values of relative peak Kv1.3 currents recorded upon co-application of the statins with flavonoids and xanthohumol.
As it is shown in Table 3, the theoretical and experimental values of the relative peak currents are comparable to each other in case of co-application of simvastatin with Ch and upon co-application of mevastatin with all the compounds except for 8-PR. In these cases, the inhibitory effects of the statins and flavonoids can be considered as additive. However, in case of co-application of both statins with 8-PR and simvastatin with 6-PR, the experimental values of relative peak currents are significantly lower than the theoretical ones (Table 3). In such cases, the inhibitory effects of the statins co-applied with flavonoids are rather synergistic than additive [26]. On the other hand, in case of co-application of simvastatin with X and Ac, the experimental values of relative peak currents are significantly higher than predicted by the theoretical model (Table 3). In these cases, the inhibitory effect exerted on the channels upon co-application of the compounds is comparable to the effect exerted by simvastatin applied alone; thus, no additivity is observed [26].
Additive or synergistic inhibitory effects exerted on Kv1.3 channels upon co-application of statins with flavonoids may be related to augmented anti proliferative and pro-apoptotic effects exerted by these compounds on Kv1.3 channel-expressing cancer cells.
Recently published data have shown that co-application of the statins simvastatin and mevastatin with the flavonoids, except for Ac, and xanthohumol leads to a significant reduction of viability of Jurkat T cells, revealed by a decrease of the IC50 value [26]. The values of IC50 upon application of the flavonoids and xanthohumol alone and in a combination with the statins are depicted in Table 4.
| Flavonoid | Applied alone | Co-applied with simvastatin | Co-applied with mevastatin |
|---|---|---|---|
| 8-PR | > 40 μM | 26.9 μM | 7.1 μM |
| 6-PR | > 40 μM | 38.9 μM | 34.8 μM |
| Ch | 26.2 μM | 10.8 μM | 8.3 μM |
| X | 32.5 μM | 30.8 μM | 3.8 μM |
Table 4.
Comparison of the IC50 value of decrease of viability of Jurkat T cells upon application of the flavonoids and xanthohumol alone and in combination with the statins simvastatin and mevastatin.
The most significant reduction of this value occurred upon co-application of simvastatin with Ch and mevastatin with 8-PR, Ch, and X (Table 4). More significant reduction occurred upon co-application of mevastatin with the flavonoids than when simvastatin was co-applied with these compounds (Table 4). This may be due to the fact that mevastatin exerted stronger pro-apoptotic effect on Jurkat T cells than simvastatin [26]. Interestingly, in all these cases, there was an additive or synergistic inhibitory effect of the compounds on Kv1.3 channels (Table 3). On the other hand, no significant change of the IC50 value was observed upon co-application of simvastatin with X (Table 4). This may be related to a lack of additive inhibitory effects on Kv1.3 channels upon co-application of these compounds (Table 3).
It was also shown that co-application of simvastatin with the flavonoids, except for Ac, led to a significant increase of the activity of caspase-3 [26]. Even more significant increase of activity of this enzyme was observed upon co-application of mevastatin with the flavonoids, except for Ac, and X [26]. Moreover, it was shown that the expression of an active (cleaved) form of caspase-3 was significantly higher when Jurkat T cells were treated with mevastatin in combination with Ch, X, and 8-PR [26]. These results may indicate that the reduction of viability of Jurkat T cells upon treatment of statins applied alone and in combination with flavonoids is due to the induction of apoptosis of these cells.
Finally, it was shown that co-application of the statins with the flavonoids and xanthohumol increased the percentage of Jurkat T cells with depolarized mitochondria. It is known that the loss of mitochondrial membrane potential (MMP) is one of hallmarks of induction of the mitochondrial pathway of apoptosis [4]. Thus, the increased percentage of depolarized mitochondria may indicate that Jurkat T cells treated with the statins and the statins co-applied with the flavonoids and xanthohumol undergo apoptosis that mainly occurs by activation of its intracellular (mitochondrial) pathway. The loss of MMP may be a consequence of the inhibition of mito Kv1.3 channels by lipophilic inhibitors of the channels [4]. A significant increase of percentage of the cells with depolarized mitochondria was observed when mevastatin was co-applied with all the flavonoids (including Ac) and X [26]. A significant increase of this parameter was also observed upon co-application of simvastatin with Ac and Ch. Upon co-application of the statin with 8-PR and 6-PR, the percentage of Jurkat T cells with depolarized mitochondria increased less significantly but still remarkably [26]. This may indicate that the apoptosis of Jurkat T cells upon co-application of the statins with the flavonoids occurs as a result of activation of the mitochondrial pathway of this process. The activation of apoptosis may be a result of inhibition of mito Kv1.3 channels by the mixture of compounds, such as it may occur upon the application of the statins alone (Figure 4) [26].
3. Possible further directions
As it was mentioned above, more studies have to be done to further elucidate the role of inhibition of Kv1.3 channels in anti-cancer activities of statins. First of all, more statins should be tested from the point of view of their ability to inhibit Kv1.3 channels. It is known that antiproliferative and pro-apoptotic effects on Kv1.3 channel-expressing cancer cells are shared by other statins, such as cerivastatin, fluvastatin, and atorvastatin [1]. However, influence of these statins on the activity of Kv1.3 channels still remains unknown.
Secondly, studies on antiproliferative and pro-apoptotic effects of statin inhibitors of Kv1.3 channels should be extended to Kv1.3 channel-expressing cancer cells other than Jurkat T cells. It is known that lipophilic inhibitors of Kv1.3 channels share the ability to selectively induce apoptosis of Kv1.3 channel-expressing cell lines representing various types of cancer disorders [4]. Good candidates may be human neoplastic B-CLL cells, human osteosarcoma SAOS-2 cell line, mouse melanoma B16F10 cells, pancreatic ductal adenocarcinoma (PDAC) cell lines (i.e., As PC-1, Capan-1, Panc-1, Mia PaCa 2, Bx PC-3, Colo357), and glioblastoma GL261, A172, and LN308 cells [4].
Finally, effects of co-application of other statins (e.g., cerivastatin, fluvastatin, atorvastatin) with flavonoids on the activity of Kv1.3 channels and viability of Kv1.3 channel-expressing cancer cells should be studied in detail.
4. Conclusions
In this chapter, it was shown that the statins lovastatin, mevastatin, pravastatin, and simvastatin were all inhibitors of Kv1.3 channels in normal and cancer cells. The ability to inhibit Kv1.3 channels may be shared by other statins, which exert antiproliferative and pro-apoptotic effects on Kv1.3 channel-expressing cancer cells, such as cerivastatin, fluvastatin, and atorvastatin. In order to elucidate the putative inhibitory effects of these statins on the channels, complex electrophysiological studies will have to be carried out.
It is also known that the inhibition of Kv1.3 channels may be related to antiproliferative and pro-apoptotic effects of the statins on Kv1.3 channel-expressing cancer cells. The putative mechanism was studied in a model system—human leukemic T cell line Jurkat. The mechanisms of these activities are probably complex and include both the Kv1.3 channel-dependent and the channel-independent pathways. Studies on antiproliferative and pro-apoptotic effects of the statins on other Kv1.3 channel-expressing cancer cells, such as human neoplastic B-CLL cells, human osteosarcoma SAOS-2 cell line, mouse melanoma B16F10 cells, pancreatic ductal adenocarcinoma (PDAC) cell, and glioblastoma cell lines, remain to be performed.
Acknowledgments
The author wants to express the best thanks to the co-workers Prof. Krystyna Michalak and Anna Uryga for a good and successful research co-operation.
References
- 1.
Gazerro P, Proto M, Gangemi C, Maltifano A, Ciaglia E, Pisanti S, et al. Pharmacological actions of statins: A critical appraising in the management of cancer. Pharmacological Reviews. 2012; 64 :102-146. DOI: 10.1124/pr.111.004994 - 2.
Gordon D. Statins may be a key therapeutic for COVID-19. Medical Hypotheses. 2020; 144 :110001. DOI: 10.1016/j.mehy.2020.110001 - 3.
Palko-Labuz A, Środa-Pomianek K, Wesołowska O, Kustrzewa-Susłow E, Uryga A, Michalak K. MDR reversal and pro-apoptotic effects of statins and statins combined with flavonoids in colon cancer cells. Biomedicine & Pharmacotherapy. 2019; 109 :1511-1522. DOI: 10.1016/j.biopha.2018.10.169 - 4.
Teisseyre A, Palko-Labuz A, Środa-Pomianek K, Michalak K. Voltage-gated potassium channel Kv1.3 as a target in a therapy of cancer. Frontiers in Oncology. 2019; 9 :1-9. DOI: 10.3389/fonc.2019.00933 - 5.
Gutman G, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo L, et al. International union of pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacological Reviews. 2005; 67 :473-508 - 6.
Szabo I, Bock J, Jekle A, Soddemann M, Adams C, Lang F, et al. A novel potassium channel in lymphocyte mitochondria. The Journal of Biological Chemistry. 2005; 280 (13):12790-12798 - 7.
Feske S, Wulff H, Skolnik E. Ion channels in innate and adaptive immunity. Annual Review of Immunology. 2015; 33 :291-353 - 8.
Perez-Verdaguer M, Capera J, Serrano-Novillo C, Estadella I, Sastre D, Felipe A. The voltage-gated potassium channel Kv1.3 is a promising multitherapeutic target against human pathologies. Expert Opinion on Therapeutic Targets. 2016; 20 (5):577-591 - 9.
Chandy KG, Norton R. Peptide blockers of Kv1.3 channels in T cells as therapeutics for autoimmune diseases. Current Opinion in Chemical Biology. 2017; 38 :97-107 - 10.
Serrano-Albarras A, Estadella I, Cirera-Rocosa S, Navarro-Perez FA. Kv1.3: A multifunctional channel with many pathological implications. Expert Opinion on Therapeutic Targets. 2018; 22 (2):101-105 - 11.
Bachmann M, Li W, Edwards M, Ahmad S, Patel S, Szabo I, et al. Voltage-gated potassium channels as regulators of cell death. Frontiers in Cell and Developmental Biology. 2020; 8 :1-17. DOI: 10.3389/fcell.2020.611853 - 12.
Kazama I. Targeting lymphocyte Kv1.3 channels to suppress cytokine storm in severe COVID-19: Can it be a novel therapeutic strategy? Drug Discoveries & Therapeutics. 2020; 14 :143-144. DOI: 10.5582/ddt.2020.030-46 - 13.
Felipe A, Vincente R, Villalonga N, Roura-Ferrer M, Martinez-Marmol R, Sole L, et al. Potassium channels: New targets in cancer therapy. Cancer Detection and Prevention. 2006; 30 :375-385 - 14.
Bielanska J, Hernández-Losa J, Perez-Verdaguer M, Moline T, Somoza R, Ramón y Cajal S, et al. Voltage-dependent potassium channels Kv1.3 and Kv1.5 in human cancer. Current Cancer Drug Targets. 2009; 9 :904-914 - 15.
Felipe A, Bielanska J, Comes N, Vallejo A, Roig S, Ramón y Cajal S, et al. Targeting the voltage-gated K+ channels Kv1.3 and Kv1.5 as tumor biomarkers for cancer detection and prevention. Current Medicinal Chemistry. 2012; 19 :661-674 - 16.
Comes N, Bielanska J, Vallejo-Garcia A, Serrano-Albarras A, Marruecos L, Gomez C, et al. The voltage-dependent K+ channels Kv1.3 and Kv1.5 in human cancer. Frontiers in Physiology. 2013; 4 (Article 283):1-12 - 17.
Checchetto V, Prosdocini E, Leanza L. Mitochondrial Kv1.3: A new target in cancer biology? Cellular Physiology and Biochemistry. 2019; 53 (51):52-62 - 18.
Prosdocimi E, Checchetto V, Leanza L. Targeting the mitochondrial potassium channel Kv1.3 to kill cancer cells: Drugs, strategies and new perspectives. SLAS Discovery. 2019; 24 (9):882-892. DOI: 10.1177/2472555219864894 - 19.
Kadow S, Schumacher F, Kramer M, Hessler G, Scholtysik S, Oubari S, et al. Mitochondrial Kv1.3 channels as target for treatment of multiple myeloma. Cancers. 2022; 14 :1-16. DOI: 10.3390/cancers14081955 - 20.
Li W, Wilson G, Bachmann M, Wang J, Mattarei A, Paradisi C, et al. Inhibition of mitochondrial potassium channel in combination with gemcitibane and abraxane drastically reduces pancreatic ductal adenocarcinoma in an immunocompetent orthotopic murine model. Cancers. 2022; 14 :1-23. DOI: 10.3390/cancers14112618 - 21.
Kazama I, Baba A, Muruyama Y. HMG-CoA reductase inhibitors: Pravastatin, lovastatin and simvastatin suppresses delayed rectifier K+-channel currents in murine thymocytes. Pharmacological Reports. 2014; 66 :712-717 - 22.
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high- resolution current recording from cells and cell-free membrane patches. Pfluegers Archiv. 1981; 39 :85-100 - 23.
Zhao N, Dong Q , Qian C, Li S, Wu Q , Ding D, et al. Lovastatatin blocks Kv1.3 channel in human T cells: A new mechanism to explain its immunomodulatory properties. Scientific Reports. 2015; 5 :1-16. DOI: 10.1038/srep17381 - 24.
Teisseyre A, Uryga A, Michalak K. Statins as inhibitors of voltage-gated potassium channels Kv1.3 in cancer cells. Journal of Molecular Structure. 2021; 1230 :1-8. DOI: 10.1016/j.molstruc.2021.129905 - 25.
Wang S, Ran Y, Chen X, Li C, Cheng S, Liu J. Pleiotropic effects of simvastatin on the regulation of potassium channels in monocytes. Frontiers in Pharmacology. 2020; 11 :101. DOI: 10.3389/fphar.2020.00101 - 26.
Teisseyre A, Chmielarz M, Uryga A, Środa-Pomianek K, Palko-Labuz A. Co-application of statin and flavonoids as an effective strategy to reduce the activity of voltage-gated potassium channels Kv1.3 and induce apoptosis in human leukemic T cell line Jurkat. Molecules. 2022; 27 :3227. DOI: 10.3390/molecules27103227 - 27.
Teisseyre A, Palko-Labuz A, Uryga A, Michalak K. The influence of 6-prenylnaringenin and selected non-prenylated flavonoids on the activity of Kv1.3 channels in human Jurkat T cells. The Journal of Membrane Biology. 2018; 251 :695-704. DOI: 10.1007/s00232-018-0046-7