Amplicon size concerning sample type and molecular biology methods (FFT: fresh frozen tissue, FFPET: formalin-fixed paraffin-embedded tissue).
Abstract
Helicobacter pylori (Hp) is one of the most common infections worldwide, with important implications in gastric pathology. Early diagnosis and treatment are essential for the control and prevention of gastric diseases. The role of Hp in the oral cavity has been investigated and studied for the past 30 years, with a growing interest because oral-oral transmission is one of the main routes. In patients with burning, halitosis, and lingual papillary hypertrophy (BHH) in the oral cavity, the dental plaque and lingual dorsum have been identified as Hp reservoirs for colonization. BHH is suggested as an effective marker for early diagnosis of Hp infection, which should be confirmed by molecular techniques and correlated with gastric involvement.
Keywords
- Helicobacter pylori
- oral pathology
- oral-oral transmission
- dental plaque
- lingual dorsum
- burning
- halitosis
- and lingual papillary hypertrophy (BHH)
1. Introduction
However, it was observed that the annual reoccurrence rate of Hp after eradication of successful gastric infection was 13% due to oral Hp infection, even in patients with negative urea breath test results, which indicates that the stomach Hp infection was cured in these patients. Reinfection in these patients suggested that the oral cavity is a secondary site for Hp colonization [2]. So, from these findings, three transmission routes have been proposed: oral-oral, gastric-oral, and fecal-oral.
There has been increasing interest in Hp infection in the oral cavity since the oral-oral route is one of the primary transmission ways. Early diagnosis is essential for treating and controlling Hp infection worldwide.
Besides the role of Hp in the oral cavity has been investigated and studied for the last 30 years, the results obtained to date are antagonistic, perhaps due to the various researchers’ different diagnostic methods, the study designs, the inclusion/exclusion criteria, and the selected controls. On the one hand, in patients with BHH in the oral cavity, the dental plaque and lingual dorsum have been identified as Hp reservoirs for colonization. On the other hand, the mouth was also investigated in patients with aphthous stomatitis, cancer, and Sjögren’s syndrome [3].
2. Dental plaque and saliva in periodontal disease
Dental plaque, or oral biofilm, is a translucent film that is a mixture of biotic matrix (bacteria, fungi) and intra/extracellular matrix (organic and mineral compounds), which was firmly adhered to tooth surfaces, gingival and oral epithelium, prostheses, and restorations. It is not removable with a simple rinse, and its composition varies depending on the location and maturation time. It is the direct cause of caries and periodontal disease on dental and periodontal surfaces. Dental plaque hosts various bacteria, and in the absence of good oral hygiene, it forms quickly and adheres to the surface of the teeth at the supragingival and subgingival levels.
In 1989, Kradjen and collaborators were the first to isolate Hp by culture from dental plaque in patients with Hp-positive gastric pathology in Canada, but they only found Hp in 1.4% (1/71) cases [4]. In 2001, 108 patients with gastric pathology and Hp-positive dental plaque were identified in Turkey, whose patients with good oral hygiene did not experience reoccurrences after being treated with antibiotic therapy [5]. In Iran, similarities were found between Hp strains isolated from saliva, feces, and gastric samples, indicating the possible role of saliva as a source of Hp infection. However, the diversity of Hp genotypes among the stomach, feces, and saliva in the same patient suggests that more than one Hp strain may exist in the saliva and stomach of the same patient due to coinfection or genetic variation [6]. In global studies, dental plaque has been identified as a reservoir of Hp in the oral cavity and as the gateway for bacteria entering the gastrointestinal system [7]. Proof of this was the clinical trial carried out in the gastroenterology unit of the Sisli Hamidiye Etfal Hospital in Turkey, where Hp was detected in the dental biofilm and saliva of children with gastric Hp, which allowed us to understand that both gastric reinfection and treatment failure could be due to the presence of Hp in the oral cavity [8]. In periodontal lesions, the number of bacteria increases with the development of periodontitis. Along with
The high prevalence of Hp in dental plaque has shown that the oral cavity can be an essential reservoir of Hp. Poor oral hygiene can lead to reinfection after eradication treatment. Good oral hygiene could contribute positively to the treatment of gastritis. Therefore, oral hygiene education should be provided to patients. More studies are needed to clarify the importance of oral Hp and its relationship with salivary parameters and oral hygiene status [15].
3. Other associated oral pathologies
3.1 Aphthous stomatitis
Aphthous stomatitis is characterized by erosions/ulcerations with a necrotic background, erythematous halo, and an oval shape involving mucous membranes. Its most frequent locations include the labial mucosa, buccal mucosa, mouth floor, tongue’s ventral surface, soft palate, tonsillar pillars, and alveolar gingiva [16]. Due to histological similarities with gastric ulcers, an association between Hp and recurrent aphthous stomatitis (RAS) is suggested. However, some studies using serological tests and biopsies failed to show such an association [17, 18]. On the other hand, attempts were also made to establish the role of Hp in the pathogenesis of RAS in patients with HIV, but the results were negative [19]. In addition, the association could not be demonstrated using molecular biology as a diagnostic method [20, 21, 22, 23, 24, 25].
The impact of Hp eradication on the clinical course of RAS was investigated in patients with Hp-positive gastric biopsies. It was observed that eradicating Hp significantly increased vitamin B12 levels and reduced the number of lesions in these patients. These facts could be explained by the hypothesis that Hp could potentially multiply in macrophages, dendritic cells, and epithelial cells and then indirectly lead to vitamin B deficiency, which would then cause changes in the epithelium of the tongue and buccal mucosa that would lead to mucosal bleeding and glossitis, given its essential role in DNA synthesis [26, 27].
3.2 Oral cancer
3.2.1 Oral squamous cell carcinoma
Oral squamous cell carcinoma (OSCC) constitutes 90% of oral cancer cases. Risk factors for OSCC include tobacco use, alcohol consumption, and human papillomavirus (HPV) infection, which may modify the oral cavity’s microbial composition. The oral microbiota and known OSCC risk factors appear to drive oral carcinogenesis. For instance, oral bacteria can transform alcohol into acetaldehyde, promoting mutagenesis in the oral mucosa [28].
The association between oral cancer and Hp action was hardly investigated in biopsies, swabs, and serum samples searching the bacteria, using culture, serology, histopathology, urease test, CLO test (Campylobacter-like organism test) rapid urease test, and PCR methods [29, 30]. Recent pilot studies have suggested a possible association between Hp and OSCC, but additional research in larger populations is needed to confirm and precisely quantify this potential association [31, 32].
3.2.2 Mucosa-associated lymphoid tissue (MALT) lymphoma
Isaacson and Wright, in 1983, introduced the term mucosa-associated lymphoid tissue (MALT) lymphoma, defined by the WHO as extranodal B-cell lymphoma of the marginal zone of mucosa-associated lymphoid tissue, that represented 10% of all lymphomas and 3% of gastric neoplasms. MALT lymphoma is commonly located at the gastrointestinal level [33, 34]. The causal relationship between Hp infection and gastric MALT lymphoma is supported by its high remission rate after infection resolution (>75%) [35]. Some studies have reported cases in major (parotid) and minor (labial) salivary glands in patients with Sjögren’s syndrome associated with Hp infection. Antibiotic therapy contributed to successful Hp eradication and MALT lymphoma resolution [36, 37, 38].
3.3 Burning, halitosis, and lingual papillary hypertrophy (BHH)
Halitosis refers to the abnormal odor of exhaled air from the mouth. The term derives from the Latin “halitus” (breath) and the Greek “osis” (disease) [39]. The three main diagnostic methods for halitosis are gas chromatography, organoleptic measurement, and sulfur monitoring [40]. Halitosis has been attributed to various factors, with highlighted gastric imbalances [41].
After Hp eradication therapy in patients with gastric pathology, halitosis disappeared with antibiotic treatment. It was associated with gastric-level Hp infection [42, 43, 44, 45]. Researchers suggested that halitosis could be an effective biomarker for predicting erosive changes beyond inflammation in the gastric mucosa of affected patients [46].
Oral halitosis is caused by volatile sulfur compounds (VSCs) generated by bacterial metabolism degrading sulfur in amino acids [47]. Anaerobic and predominantly gram-negative bacteria, found in the gingival sulcus, periodontal pockets, and posterior lingual dorsum, play a crucial role [48].
Patients with BHH may present with both tongue and gastric Hp infections. These patients did not respond effectively to treatment and were diagnosed with chronic candidiasis concerning lingual papillary hypertrophy. They were referred to Periodontics services for Halitosis. A considerable percentage (60%) of these patients had a history of chronic untreated gastric discomfort. Diagnosing Hp in the oral cavity must be confirmed with molecular biology, and the patient’s gastric involvement must be evaluated [54, 55].
4. Diagnosis of oral infection by Helicobacter pylori (Hp)
Some methods for diagnosing oral Hp infection analyze this bacteria’s structural and functional characteristics. Other methods evaluate the immune response it provokes in the host. Not all methods used in the stomach work in the same way. The reasons for choosing one or another diagnostic tool are based on the clinical case and the availability of these methodologies. Any of the methods used to detect Hp in the mouth requires that the patient is not under medication, both oral and general, in the last 4 weeks with simethicone, proton pump inhibitors, histamine H2 blockers, bismuth, antibiotics, antifungals, and mouthwashes. The methods can be invasive, generally based on the study of the biopsy, and non-invasive by studying samples obtained by swab, cytobrush, saliva, or blood.
4.1 Histology
The histological study of the biopsy allows us to identify the oral mucosa lesions but does not detect Hp infection. In 2001, Adler and collaborators identified the Hp genome for the first time in the biopsy of a 35-year-old male patient with halitosis and burning mouth, accompanied by dyspepsia, who at the time of consultation presented hyperplasia of posterior filiform papillae with negative mycological culture and biopsy with active chronic gastritis due to Hp. A partial biopsy of the affected lingual area was performed for histopathological study and determination of Hp by PCR (see Section 5.4). The histopathological study showed marked epithelial hyperplasia, papillomatosis, acanthosis, and hyperparakeratosis. The underlying chorion presented an inflammatory infiltrate composed predominantly of lymphocytes and attached to the surface of the epithelium; there was a thick bacterial plaque with abundant coccoid elements. With the Giemsa technique, spiral bacteria adhered to the epithelial surface were identified, with morphology similar to that of Hp classically described in gastric biopsies (Figure 1). Identification of microorganisms by this method requires confirmatory genomic evaluation (see Section 5.2) [56].

Figure 1.
(A) Epithelial hyperplasia, papillomatosis, acanthosis, and hyperparakeratosis are observed. In the underlying chorion, an inflammatory infiltrate is composed predominantly of lymphocytes. Attached to the surface of the epithelium was a bacterial plaque with abundant coccoid elements (hematoxylin-eosin - HE). (B) Spiral bacteria adhering to the epithelial surface are individualized. The morphology of these elements is similar to that of the Hp described in gastric biopsies (Giemsa) [
4.2 Culture
The culture of Hp is a specific method whose sensitivity depends on variables such as sample collection, transportation, storage, culture media used, and incubation conditions (percentage of CO2 and humidity). Once the sample is obtained, it must be placed in a carbonated Stuart transport medium. This method allows for determining sensitivity to antimicrobials, characterizing virulence factors, and typing strains for epidemiological purposes. This technique is inappropriate in dental plaque and the lingual dorsum due to the presence of coccoid forms of Hp and abundant other accompanying pathogens [57].
4.3 Rapid urease test (RUT)
The rapid urease test is a qualitative test used to detect the presence of Hp by measuring the activity of the bacteria’s urease enzyme. The sample is placed in a urea tube containing a pH indicator. Despite being a rapid method for identifying Hp, it would not be specific in the oral cavity because other bacteria have urease activity [58].
4.4 Serological tests
Hp provokes a local and systemic immune response. Serological tests are based on detecting specific circulating antibodies in response to bacterial antigens in saliva or serum. Several serological methods have been developed for detecting Hp, such as bacterial agglutination, complement fixation, indirect immunofluorescence, and enzyme immunoassay techniques. The most used approach is enzyme immunoassay (EIA) and enzyme-linked immunosorbent assay (ELISA) because it is quantitative, allowing treatment monitoring. However, its value in diagnosing Hp infection in the mouth must be interpreted cautiously since a positive result does not determine the ubiquity of Hp. Another local option is to study antibodies in saliva, which is an easy sample to obtain, but the main disadvantage is the low concentration of antibodies in them [59].
4.5 Breath test (urea breath test—UBT)
The breath test or exhaled air test is a non-invasive indirect method. It is based on detecting the presence of Hp urease by taking samples of the patient’s breath with C13 (non-radioactive), the first in a basal state and the second 30 minutes after ingesting a solution with urea isotopically labeled or only one sample with C14 (radioactive). If Hp is in the mouth and/or stomach, it hydrolyzes urea thanks to its urease, and labeled CO2 (C13 or C14) is released, absorbed, diffused into the blood, and released with the breath. A non-radioactive method has similar sensitivity but requires a mass spectrometer to measure. The breath test indicates an active infection because urease is produced in the viable organism. However, its value in diagnosing Hp infection in the mouth must be interpreted cautiously since a positive result does not determine the ubiquity of Hp, and the breath test must not ever be used for oral HP diagnosis due to the presence of other urease-positive microbes [59].
5. Molecular pathology of Helicobacter pylori (Hp)
Hp has a small 1.6-Mbp genome compared to many other pathogenic bacteria, consisting of a single circular chromosome that encodes about 1600 proteins. The Hp core genome consists of approximately 1100 genes. The Hp genome is different between strains and even within the bacteria present in an individual’s stomach, resulting from the unusual combination of very high rates of mutation and recombination. This high frequency of mutations can be explained by the lack of proofreading function in the DNA polymerase in combination with the pro-mutagenic properties of its DNA. The diversity of DNA sequences can spread rapidly through Hp populations due to recombination between strains. Hp can be updated extracellularly because its DNA can be recombined, allowing HP to adapt its genome to new environments exceptionally efficiently [60].
5.1 Molecular technique
5.1.1 Polymerase chain reaction (PCR)
Kary B. Mullis obtained The Nobel Prize in Chemistry in 1993 for inventing the PCR process, in which a small amount of DNA can be copied in large quantities over a short period. PCR is based on synthesizing new DNA strands complementary to the template strand present using DNA polymerase that can add a nucleotide to a pre-existing 3′-OH group that will be offered by a primer with complementary sequence to the region of interest that will be mandatorily necessary to add the first nucleotide. Two primers flanking the sequence of interest are needed so that at the end of the PCR reaction, the particular sequence accumulates into billions of copies (amplicons) since the PCR reaction generates copies of the target sequence exponentially (Figure 2) [61, 62].
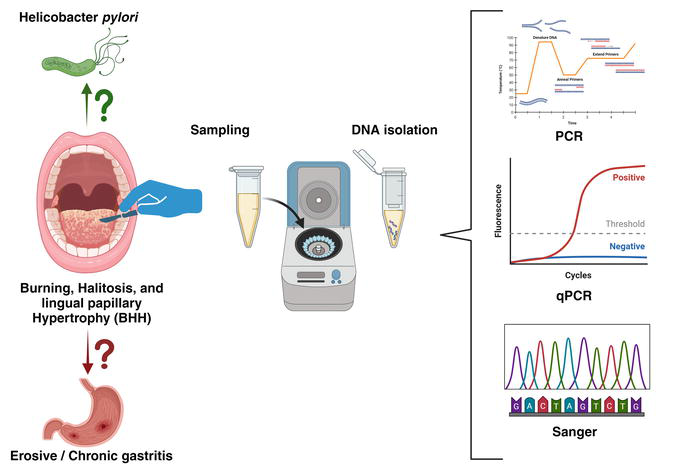
Figure 2.
Halitosis is an effective sign for early diagnosis of
5.1.2 Real-time quantitative PCR (qPCR) assays
During the PCR reaction, if one wants to know the initial amount of the target sequence contained in the sample, one must measure the amplification in the exponential phase of the PCR because only in that phase is there a linear relationship between reactive and product. Like any enzymatic reaction, the target sequence ceases to amplify due to the product accumulation, and a “plateau effect” appears. At this point, the endpoint PCR is measured because it is the maximum concentration point of amplicons that the reaction can obtain. This attribute of PCR makes real-time quantitative PCR (qPCR) necessary, which amplifies a targeted DNA molecule during the PCR process (Figure 2). There exist at least two methods for detecting PCR products: non-specific fluorescent dyes that bind double-stranded DNA molecules by intercalating between the DNA bases and sequence-specific fluorescence resonance energy transfer probes that only see the amplicon hybridized with its complementary sequence (TaqMan) [63].
5.1.3 Sanger capillary sequencing
Sanger sequencing was first developed by Frederick Sanger and colleagues in 1977 and has become the most widely used sequencing method for over 40 years. It is a DNA sequencing method based on the selective incorporation of chain-terminating dideoxynucleotides (ddNTPs) by DNA polymerase during in vitro DNA replication [63]. DNA polymerase enzyme adds a nucleotide to a pre-existing 3′-OH group of the primer, either deoxynucleotide triphosphates (dNTPs: dATP, dGTP, dCTP, dTTP) or chain-terminating dideoxynucleotide triphosphates (ddNTPs: ddATP, ddGTP, ddCTP, ddTTP) in rate-limiting concentrations which stop the elongation reaction as the ddNTPs are incorporated, resulting in distinguishable DNA fragments of various lengths. A high-quality DNA template is mandatory for sequence analysis. Therefore, storage duration, temperature, and fixatives have an essential impact on the recovery [64]. Sequencing procedures require DNA or RNA amplicons that are longer than those used for qPCR (150 bp) for optimal results (Figure 2). Sanger method remains widely used for smaller-scale projects and validation of next-generation sequencing (NGS).
5.1.4 Next-generation sequencing (NGS)
Large-scale parallel sequencing (next-generation sequencing, NGS) is a DNA sequencing technology revolutionizing genomic amplification. NGS can sequence the whole human genome within a single day. Over the last few years, massively parallel sequencing has rapidly evolved and transitioned into molecular pathology routine laboratories. This is an exciting platform for simultaneously analyzing multiple genes with low input material. The spectrum of DNA variation in a human genome comprises small base changes (substitutions), insertions and deletions of DNA, large genomic deletions of exons or whole genes, and rearrangements, such as inversions and translocations [63]. This methodology could be used to analyze Hp resistance in an automated way, as well as the classification of bacterial strains.
5.2 Tissue sampling
The central dogma of molecular biology explains the flow of genetic information within a biological system: “DNA makes RNA, and RNA makes protein,” the analysis of each biomolecule is called genomics, transcriptomics, and proteomics, respectively. This chapter focused on a Hp genomic approach, and it should be emphasized that genomic research is an indirect viability measure because it does not imply an active bacterial infection. Therefore, a genomic approach performed from fresh frozen tissue (FFT) complements the clinical lesion observed, and served from formalin-fixed paraffin-embedded tissue (FFPET) complements the histological characteristics.
5.2.1 Fresh frozen tissue (FFT)
Fresh frozen tissue (FFT) is the “gold standard” type of biosample for PCR and especially for sequencing. However, frozen tissue collection is generally not feasible because clinical workflows provide formalin-fixed material that, unfortunately, allows less DNA to be obtained, which is usually more degraded than in fresh samples, which produces sequencing artifacts associated with the fixation process [64].
5.2.2 Formalin-fixed paraffin-embedded tissue (FFPET)
Formalin-fixed, paraffin-embedded tissue (FFEPT) block is the “gold standard” method of human tissue preservation for diagnosis. The fixation of tissue specimens by formalin has been used for almost 120 years. A formalin concentration of 10% was suggested, which is equivalent to a final formaldehyde concentration of 4% [65]. Processing samples in this manner has several advantages; on the one hand, it mitigates risks of infectious agents that may be present in the fresh material. On the other hand, it ensures the preservation of the architectural components of the tissue, and it allows, through thin sections, to examine the architecture of the tissue using simple dyes, such as hematoxylin and eosin [66].
Fixation damages the structure of DNA and thus negatively affects subsequent PCR. For example, tissues fixed for 8 days can amplify 536-bp fragments, and for 30 days, only 268-bp [67]. Due to this fragmentation, quantification before performing a molecular technique was mandatory. Quantification and purity evaluation of nucleic acids are generally performed by spectrophotometric analysis or ultraviolet fluorescence tagging. Ultraviolet wavelength light (260 nm) absorption is directly related to the sample’s nucleic acid concentration. Purity assessments are employed to detect (i) contaminating proteins with a peak absorption of 280 nm, particularly from the aromatic amino acids, with a sample’s 260 nm/280 nm absorbance ratio > 1.8 ratio; (ii) other contaminants with a peak absorption on 230 nm, particularly from carbohydrates, phenol, guanidine, and glycogen, with a sample’s 260 nm/230 nm absorbance ratio of 2.0–2.2 [64].
5.3 PCR amplicon size
Ideal amplicon length/size depends on many variables and design preferences. For standard PCR, scientists generally design amplicons between 200 and 1000 bp. However, for quantitative PCR, expected amplicons range from 75 to 150 bp. But, as mentioned above, fixation breaks the genetic material, and PCR performed in FFPET needs a design of specific-sequence primers that flank targets with molecular weights less than 300 bp.
There are different genetic targets of Hp. The most used target gene in the literature was the 16S rRNA, but they also used cagA, vacA genes, and urease A [3]. The selection depends on the amplified fragment’s sensitivity and specificity according to the primers’ design that defines a specific size, allowing different amplification methods depending on the patient’s sample (Table 1).
| FFT | FFPET | |
|---|---|---|
| End point PCR | 200–1000 | <300 bp |
| Quantitative PCR | <150 bp | |
| Sanger sequencing | >150 bp | >150 bp |
Table 1.
5.4 Helicobacter pylori molecular approach
Based on the test, laboratory equipment available, and the patient clinical characteristics, the optimal diagnostic approach requires weighing several factors.
As mentioned above, Adler et al. described a case report in 2001 that, for the first time, identified Hp in BHH. The FFPET biopsy was histologically analyzed with HE and Giemsa staining, while Hp DNA was detected by PCR [56]. The primers used for gastric biopsies in FFPET were employed in this first oral case. The Hp genome was studied with a semiquantitative molecular technique (PCR), with primers homologous to a region that codes for the species-specific antigen. Their nucleotide sequence was HP3: 5′-TGGCGTGTCTATTGACAGCGAGC-3′ and HP4: 5′-CCTGCTGGGCATACTTCACCATG-3′, identified with 474–496 and 776–754 residue peptides, respectively, that flank a 298-bp amplicon [68]. Negative, positive, and blank controls were used. On the other hand, a second PCR was carried out under similar conditions for a 110 bp region of the human beta-globin gene, as amplification control of a DNA patient using the oligonucleotide primers PC03 and PC04 [69]. All these PCRs were analyzed with 9% polyacrylamide gel electrophoresis in TBE 1X buffer (Tris-Boric-EDTA) and visualized with ethidium bromide under ultraviolet light. As the fragment was more significant than 150 bp (Table 1), two-way Sanger sequencing could be performed and aligned by basic local alignment search tool (BLAST) with the Hp genome. By 2005, Adler et al. increased the study population. They studied extensively patients with BHH by molecular methods, intending to confirm the relationship between BHH and Hp reliably [54]. DNA was purified from 7-μm sections of the lingual mucosa biopsies of the affected site, which were formalin-fixed paraffin-embedded. The same protocol as in 2001 was followed. Still, the positive results of Hp were confirmed with a second PCR, with sequence-specific primers that amplified a 109 bp region of the 16Sr RNA gene HP1: 5′-CTGGAGAGACTAAGCCCTCC-3′ and HP2: 5′-ATTACTGACGCTGATTGTGC-3′ [70]. This study of two different genomic targets confirmed the linkage of BHH with the presence of the Hp genome.
By 2014, Adler’s group decided to convert the method to a less invasive test by sampling the lingual dorsum by scraping and performing PCR from fresh tissue to increase sensitivity, specificity, and speed of results [3]. For this purpose, primers were used to allow qualitative end-point PCR assays but with the possibility of using the same design in a real-time PCR. The primer that amplifies a 114-bp fragment corresponding to the 16S rRNA gene of Hp was 16S2-F:5′-CGCTAAGAGATCAGCCTATGTCC-3′ and 16SB2-R: 5′-CCGTGTTCTCAGTTCCAGTGTGT-3′ (GenBank: U00679.1) [71]. Quantifying the bacterial inoculum allows clinical monitoring of patients and evaluation of the response to local treatment. However, quantitative methods should be performed on fresh samples.
6. Treatment with effectiveness in the oral cavity
Before the discovery of Hp in the 1980s, peptic ulcer was considered a chronic disease: “Once there is an ulcer, there is always an ulcer,” but once the bacterial infection was described as the cause of the pathogen, it was transformed into a unique disease, since healing Hp infection prevents ulcer recurrence and the development of new peptic ulcers. Despite the recognition that Hp is the leading cause of atrophic gastritis, the hypothesis that its eradication could prevent and reduce the incidence of gastric cancer was slowly accepted. More than a decade passed after Hp was defined as a human carcinogen for acceptance that curing the infection also reduces the incidence and prevents the development of gastric cancer [72].
Effective Hp therapy may include an antisecretory drug, antimicrobials to reduce the chances of survival of pre-existing resistant subpopulations, and bismuth. The most used therapy is the 14-day triple therapy consisting of a combination of three drugs, a proton pump inhibitor (PPI), and two antibiotics, which can be amoxicillin and clarithromycin, one of which can be replaced in particular cases by metronidazole. Other options are quadruple therapy with bismuth, triple therapies with levofloxacin, or 7-day triple therapies with vonoprazan. Resistance to these combinations has been described, resulting in a lower effectiveness rate [73, 74]. After successful treatment, the oral cavity is a reservoir for gastric re-infection [75]. In Korea, applying a general eradication regimen with red ginseng supplementation at the local level showed eradication rates of 93% [76]. Adler’s group, based on the case report of a male patient with Hp infection, both in the mouth (BHH) and in the stomach, established triple therapy in their patients at a general level and added oral hygiene teaching, periodontal treatment, cleaning the tongue with a gauze soaked with mouthwash and topical application of metronidazole gel on the dorsum of the tongue, with a decrease in the recurrence rate of Hp after eradication [56, 77, 78]. The literature on clarithromycin’s effects on the lingual surface is old and scarce, and studies that address this treatment in this organ are needed. Still, the use of systemic antibiotics, such as clarithromycin, has been reported to be associated with adverse effects in the oral cavity, such as glossitis that exacerbates BHH syndrome (Figure 3) [79].

Figure 3.
(A) Patient with BHH and Hp-positive gastritis, confirmed by PCR technique. (B) Post triple therapy and local medication in the tongue. The evolution of BHH was observed after 7 days [
The diagnosis of candidiasis should be made by mycological culture and fungigram to confirm the species which co-infected the lesion (

Figure 4.
Diagnosis and treatment algorism of
Infection control includes preventive environmental and oral hygiene measures and regular dental and gastroenterological consultations. Good oral hygiene could contribute positively to the treatment of gastritis. Therefore, oral hygiene education should be provided to patients. More studies are needed to clarify the importance of oral Hp and its relationship with salivary parameters and oral hygiene status [15]. It has been shown that the prevalence of Hp is 20% in urban areas. Still, in rural areas, it exceeds 60%, suggesting that sociocultural and economic factors could determine these differences because they reflect different levels of hygiene, sanitation, living density, and education [78]. However, BHH syndrome does not improve only with good hygiene; it is necessary to administer additional local pharmacological therapy and have periodic consultations with the specialist.
7. Helicobacter pylori challenges
Hp infection, which affects approximately half of the world’s population, remains a severe public health problem. Li et al. identified a lower prevalence of Hp infection in younger people, high-income countries, or countries with high levels of universal health coverage. Studies based on serological diagnostic methods generally reported higher Hp prevalence than studies based on non-serological methods (53% vs. 41%) [80]. Hp leads to several gastric pathologies, including inflammation, gastroduodenal ulcers, and malignancies. Early detection and treatment are crucial to preventing the spread of the infection [81].
Precision medicine is an innovative approach to disease prevention and treatment that considers differences in people’s genes, injuries, environments, and lifestyles to target the right therapies to the right patients at the right time [82]. In this new approach to Hp infection medicine, the challenges could be:
To identify germline pathogenic variants in homologous-recombination genes (ATM, BRCA1, BRCA2, CDH1, MLH1, MSH2, MSH6, and PALB2) by NGS, considering that individuals with Hp infection and this germline altered genes had a higher cumulative risk of gastric cancer than non-carriers infected with Hp (45% vs. 14%) [83].
To confirm if Hp reduces treatment efficacy with immune checkpoint inhibitors in malignant diseases before developing a vaccine for preventive and therapeutic purposes that might consider multiple epitopes to direct the immune response toward essential Hp functions such as epithelial cell adherence, proliferation, and survival in the specific gastric niche [60].
To incorporate nanoparticles and microparticles in clinical trials to overcome the barriers of the oral route, physicochemical inconveniences, and lack of selectivity of current therapy. Standard oral treatment fails in 20% of cases since the obstacles of the oral route decrease the bioavailability of antibiotics, increasing the rates of resistance [84].
To develop a new treatment against the redox switch protein HP1021, which controls the transcription of 497 genes, including 407 genes related to response to oxidative stress, that allow Hp to combat stress factors, including reactive oxygen species (ROS) [85].
8. Conclusions
Hp infection is one of the most common infections in the world, and early diagnosis and treatment are essential for its control. One of the strategies for early diagnosis is to detect Hp in BHH, especially in patients with gastric pathologies. The diagnostic method of choice for Hp infection in the mouth is genome detection by PCR. Infection control consists of preventive environmental and oral hygiene measures and regular dental and gastroenterological consultations. Therapeutic measures should consider the joint treatment of oral and gastric pathology, trying in the future to approach precision medicine with massive genetic analysis of the resistance to treatments that the bacterium acquires rapidly due to its high mutation rate, as well as the possible identification of germline variants of the host that increase the risk of gastric cancer in patients already infected.
Acknowledgments
We thank Drs. Elsner, Avagnina, and Fresno for their unconditional support, as well as the staff and patients of the Stomatological Clinic of the University of Buenos Aires (UBA).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.
Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984; 1 :1311-1135. DOI: 10.1016/s0140-6736(84)91816-6 - 2.
Yee JK. Helicobacter pylori colonization of the oral cavity: A milestone discovery. World Journal of Gastroenterology. 2016;22 :641-648. DOI: 10.3748/wjg.v22.i2.641 - 3.
Adler I, Muiño A, Aguas S, Harada L, Diaz M, Lence A, et al. Helicobacter pylori and oral pathology: Relationship with the gastric infection. World Journal of Gastroenterology. 2014;20 :9922-9935. DOI: 10.3748/wjg.v20.i29.9922 - 4.
Krajden S, Fuksa M, Anderson J, Kempston J, Boccia A, Petrea C, et al. Examination of human stomach biopsies, saliva, and dental plaque for Campylobacter pylori . Journal of Clinical Microbiology. 1989;27 :1397-1398. DOI: 10.1128/jcm.27.6.1397-1398.1989 - 5.
Avcu N, Avcu F, Beyan C, Ural AU, Kaptan K, Ozyurt M, et al. The relationship between gastric-oral Helicobacter pylori and oral hygiene in patients with vitamin B12-deficiency anemia. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2001;92 :166-169. DOI: 10.1067/moe.2001.113589 - 6.
Momtaz H, Souod N, Dabiri H, Sarshar M. Study of Helicobacter pylori genotype status in saliva, dental plaques, stool and gastric biopsy samples. World Journal of Gastroenterology. 2012;18 :2105-2111. DOI: 10.3748/wjg.v18.i17.2105 - 7.
Wang XM, Yee KC, Hazeki-Taylor N, Li J, Fu HY, Huang ML, et al. Oral Helicobacter pylori , its relationship to successful eradication of gastricH. pylori and saliva culture confirmation. Journal of Physiology and Pharmacology. 2014;65 :559-566 - 8.
Aksit Bıcak D, Akyuz S, Kıratlı B, Usta M, Urganci N, Alev B, et al. The investigation of Helicobacter pylori in the dental biofilm and saliva samples of children with dyspeptic complaints. BMC Oral Health. 2017;17 :67-78. DOI: 10.1186/s12903-017-0361-x - 9.
Asikainen S, Chen C, Slots J. Absence of Helicobacter pylori in subgingival samples determined by polymerase chain reaction. Oral Microbiology and Immunology. 1994;9 :318-320. DOI: 10.1111/j.1399-302x.1994.tb00079.x - 10.
Dye BA, Kruszon-Moran D, McQuillan G. The relationship between periodontal disease attributes and Helicobacter pylori infection among adults in the United States. American Journal of Public Health. 2002;92 :1809-1815. DOI: 10.2105/ajph.92.11.1809 - 11.
Agarwal S, Jithendra KD. Presence of Helicobacter pylori in subgingival plaque of periodontitis patients with and without dyspepsia, detected by polymerase chain reaction and culture. Journal of Indian Society of Periodontology. 2012;16 :398-403. DOI: 10.4103/0972-124X.100919 - 12.
Bouziane A, Ahid S, Abouqal R, Ennibi O. Effect of periodontal therapy on prevention of gastric Helicobacter pylori recurrence: A systematic review and meta-analysis. Journal of Clinical Periodontology. 2012;39 :1166-1173. DOI: 10.1111/jcpe.12015 - 13.
Liu Y, Li R, Xue X, Xu T, Luo Y, Dong Q , et al. Periodontal disease and Helicobacter pylori infection in oral cavity: A meta-analysis of 2727 participants mainly based on Asian studies. Clinical Oral Investigations. 2020l;24 :2175-2188. DOI: 10.1007/s00784-020-03330-4 - 14.
Dane A, Gurbuz T. Clinical comparative study of the effects of Helicobacter pylori colonization on oral health in children. Pakistan Journal of Medical Sciences. 2016;32 :969-973. DOI: 10.12669/pjms.324.10034 - 15.
Anand PS, Kamath KP, Anil S. Role of dental plaque, saliva and periodontal disease in Helicobacter pylori infection. World Journal of Gastroenterology. 2014;20 :5639-5653. DOI: 10.3748/wjg.v20.i19.5639 - 16.
Edward W. Odell Cawson's Essentials of Oral Pathology and Oral Medicine. 9th ed. Elsevier Health Sciences; 2017. pp. 253-281. ISBN 9780702049811 - 17.
Riggio MP, Lennon A, Wray D. Detection of Helicobacter pylori DNA in recurrent aphthous stomatitis tissue by PCR. Journal of Oral Pathology & Medicine. 2000;29 :507-513. DOI: 10.1034/j.1600-0714.2000.291005.x - 18.
Porter SR, Barker GR, Scully C, Macfarlane G, Bain L. Serum IgG antibodies to Helicobacter pylori in patients with recurrent aphthous stomatitis and other oral disorders. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 1997;83 :325-328. DOI: 10.1016/s1079-2104(97)90237-7 - 19.
Leimola-Virtanen R, Happonen RP, Syrjänen S. Cytomegalovirus (CMV) and Helicobacter pylori (HP) found in oral mucosal ulcers. Journal of Oral Pathology & Medicine. 1995;24 :14-47. DOI: 10.1111/j.1600-0714.1995.tb01123.x - 20.
Birek C, Grandhi R, McNeill K, Singer D, Ficarra G, Bowden G. Detection of Helicobacter pylori in oral aphthous ulcers. Journal of Oral Pathology & Medicine. 1999;28 :197-203. DOI: 10.1111/j.1600-0714.1999.tb02024.x - 21.
Victória JM, Kalapothakis E, Silva Jde F, Gomez RS. Helicobacter pylori DNA in recurrent aphthous stomatitis. Journal of Oral Pathology & Medicine. 2003;32 :219-223. DOI: 10.1034/j.1600-0714.2003.00136.x - 22.
Iamaroon A, Chaimano S, Linpisarn S, Pongsiriwet S, Phornphutkul K. Detection of Helicobacter pylori in recurrent aphthous ulceration by nested PCR. Journal of Oral Science. 2003;45 :107-110. DOI: 10.2334/josnusd.45.107 - 23.
Fritscher AM, Cherubini K, Chies J, Dias AC. Association between Helicobacter pylori and recurrent aphthous stomatitis in children and adolescents. Journal of Oral Pathology & Medicine. 2004;33 :129-132. DOI: 10.1111/j.0904-2512.2004.00074.x - 24.
Mansour-Ghanaei F, Asmar M, Bagherzadeh AH, Ekbataninezhad S. Helicobacter pylori infection in oral lesions of patients with recurrent aphthous stomatitis. Medical Science Monitor. 2005;11 :576-579 - 25.
Gomes CC, Gomez RS, Zina LG, Amaral FR. Recurrent aphthous stomatitis and Helicobacter pylori . Medicina Oral, Patología Oral y Cirugía Bucal. 2016;21 :e187-e191. DOI: 10.4317/medoral.20872 - 26.
Taş DA, Yakar T, Sakalli H, Serin E. Impact of Helicobacter pylori on the clinical course of recurrent aphthous stomatitis. Journal of Oral Pathology & Medicine. 2013;42 :89-94. DOI: 10.1111/j.1600-0714.2012.01197.x - 27.
Gao Y, Gupta N, Abdalla M. Recurrent aphthous stomatitis improved after eradication therapy for Helicobacter pylori . Case Reports in Gastrointestinal Medicine. 2021;2021 :5543838. DOI: 10.1155/2021/5543838 - 28.
Chen J, Domingue JC, Sears CL. Microbiota dysbiosis in select human cancers: Evidence of association and causality. Seminars in Immunology. 2017; 32 :25-34. DOI: 10.1016/j.smim.2017.08.001 - 29.
Grandis JR, Perez-Perez GI, Yu VL, Johnson JT, Blaser MJ. Lack of serologic evidence for Helicobacter pylori infection in head and neck cancer. Head & Neck. 1997;19 :216-218. DOI: 10.1002/(sici)1097-0347(199705)19:3<216::aid-hed9>3.0.co;2-5 - 30.
Meng X, Wang Q , He C, Chen M, Liu J, Liu W, et al. An inverse association of Helicobacter pylori infection with oral squamous cell carcinoma. Journal of Oral Pathology & Medicine. 2016;45 :17-22. DOI: 10.1111/jop.12324 - 31.
Dayama A, Srivastava V, Shukla M, Singh R, Pandey M. Helicobacter pylori and oral cancer: Possible association in a preliminary case control study. Asian Pacific Journal of Cancer Prevention. 2011;12 :1333-1336 - 32.
Mohtasham N, Saghravanian N, Zare R, Saghafi S, Ghazi N, Mohajertehran F, et al. Tumor tissue Helicobacter pylori and human papillomavirus infection in head and neck squamous cell carcinoma patients and association with clinicopathological indices: A cross-sectional medical survey. Dental Research Journal (Isfahan). 2022;19 :8. DOI: 10.4103/1735-3327.336693 - 33.
Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983; 52 :1410-1406. DOI: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3 - 34.
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee meeting—Airlie House, Virginia, November, 1997. The Hematology Journal. 2000; 1 :53-66. DOI: 10.1038/sj.thj.6200013 - 35.
Stolte M, Eidt S. Healing gastric MALT lymphomas by eradicating H pylori ? Lancet. 1993;342 :568. DOI: 10.1016/0140-6736(93)91404-a - 36.
Nishimura M, Miyajima S, Okada N. Salivary gland MALT lymphoma associated with Helicobacter pylori infection in a patient with Sjögren's syndrome. The Journal of Dermatology. 2000;27 :450-452. DOI: 10.1111/j.1346-8138.2000.tb02204.x - 37.
Iwai H, Nakamichi N, Nakae K, Konishi M, Inaba M, Hoshino S, et al. Parotid mucosa-associated lymphoid tissue lymphoma regression after Helicobacter pylori eradication. The Laryngoscope. 2009;119 :1491-1494. DOI: 10.1002/lary.20258 - 38.
Keszler A, Adler LI, Gandolfo MS, Masquijo Bisio PA, Smith AC, Vollenweider CF, et al. MALT lymphoma in labial salivary gland biopsy from Sjögren syndrome: Importance of follow-up in early detection. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology. 2013; 115 :e28-e33. DOI: 10.1016/j.oooo.2012.07.481 - 39.
Tangerman A. Halitosis in medicine: A review. International Dental Journal. 2002; 52 :201-306. DOI: 10.1002/j.1875-595x.2002.tb00925.x - 40.
Yaegaki K, Coil JM. Examination, classification, and treatment of halitosis; clinical perspectives. Journal of the Canadian Dental Association. 2000; 66 :257-261 - 41.
Delanghe G, Ghyselen J, van Steenberghe D, Feenstra L. Multidisciplinary breath-odour clinic. Lancet. 1997; 350 :187. DOI: 10.1016/S0140-6736(05)62354-9 - 42.
Tiomny E, Arber N, Moshkowitz M, Peled Y, Gilat T. Halitosis and Helicobacter pylori . A possible link? Journal of Clinical Gastroenterology. 1992;15 :236-237. DOI: 10.1097/00004836-199210000-00013 - 43.
Ierardi E, Amoruso A, La Notte T, Francavilla R, Castellaneta S, Marrazza E, et al. Halitosis and Helicobacter pylori : A possible relationship. Digestive Diseases and Sciences. 1998;43 :2733-2737. DOI: 10.1023/a:1026619831442 - 44.
Katsinelos P, Tziomalos K, Chatzimavroudis G, Vasiliadis T, Katsinelos T, Pilpilidis I, et al. Eradication therapy in Helicobacter pylori -positive patients with halitosis: Long-term outcome. Medical Principles and Practice. 2007;16 :119-123. DOI: 10.1159/000098364 - 45.
Moshkowitz M, Horowitz N, Leshno M, Halpern Z. Halitosis and gastroesophageal reflux disease: A possible association. Oral Diseases. 2007; 13 :581-585. DOI: 10.1111/j.1601-0825.2006.01341.x - 46.
Yoo SH, Jung HS, Sohn WS, Kim BH, Ku BH, Kim YS, et al. Volatile sulfur compounds as a predictor for esophagogastroduodenal mucosal injury. Gut and Liver. 2008; 2 :113-118. DOI: 10.5009/gnl.2008.2.2.113 - 47.
Kazor CE, Mitchell PM, Lee AM, Stokes LN, Loesche WJ, Dewhirst FE, et al. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. Journal of Clinical Microbiology. 2003; 41 :558-563. DOI: 10.1128/JCM.41.2.558-563.2003 - 48.
Rosenberg M. Clinical assessment of bad breath: Current concepts. Journal of the American Dental Association (1939). 1996; 127 :475-382. DOI: 10.14219/jada.archive.1996.0239 - 49.
De Boever EH, Loesche WJ. Assessing the contribution of anaerobic microflora of the tongue to oral malodor. Journal of the American Dental Association (1939). 1995; 126 :1384-1393. DOI: 10.14219/jada.archive.1995.0049 - 50.
Scully C, el-Maaytah M, Porter SR, Greenman J. Breath odor: Etiopathogenesis, assessment and management. European Journal of Oral Sciences. 1997; 105 :287-293. DOI: 10.1111/j.1600-0722.1997.tb00242.x - 51.
Riggio MP, Lennon A, Rolph HJ, Hodge PJ, Donaldson A, Maxwell AJ, et al. Molecular identification of bacteria on the tongue dorsum of subjects with and without halitosis. Oral Diseases. 2008; 14 :251-258. DOI: 10.1111/j.1601-0825.2007.01371.x - 52.
Siavoshi F, Saniee P. Vacuoles of Candida yeast as a specialized niche forHelicobacter pylori . World Journal of Gastroenterology. 2014;20 :5263-5273. DOI: 10.3748/wjg.v20.i18.5263 - 53.
Gall-Troselj K, Mravak-Stipetić M, Jurak I, Ragland WL, Pavelić J. Helicobacter pylori colonization of tongue mucosa—Increased incidence in atrophic glossitis and burning mouth syndrome (BMS). Journal of Oral Pathology & Medicine. 2001;30 :560-563. DOI: 10.1034/j.1600-0714.2001.300909.x - 54.
Adler I, Denninghoff VC, Alvarez MI, Avagnina A, Yoshida R, Elsner B. Helicobacter pylori associated with glossitis and halitosis. Helicobacter. 2005;10 :312-317. DOI: 10.1111/j.1523-5378.2005.00322.x - 55.
Zhao Y, Gao X, Guo J, Yu D, Xiao Y, Wang H, et al. Helicobacter pylori infection alters gastric and tongue coating microbial communities. Helicobacter. 2019;24 :e12567. DOI: 10.1111/hel.12567 - 56.
Adler I, Denninghoff V, Alvarez MI, Pecollo J, Neffen E, Avagnina A, et al. Infeccion intrabucal por helicobacter en paciente de sexo masculino. Revista del Ateneo Argentino de Odontología. 2001; 40 :10-15 - 57.
Allaker RP, Young KA, Hardie JM, Domizio P, Meadows NJ. Prevalence of Helicobacter pylori at oral and gastrointestinal sites in children: Evidence for possible oral-to-oral transmission. Journal of Medical Microbiology. 2002;51 :312-317. DOI: 10.1099/0022-1317-51-4-312 - 58.
McNicholl AG, Ducons J, Barrio J, Bujanda L, Forné-Bardera M, Aparcero R, et al. Accuracy of the ultra-rapid urease test for diagnosis of Helicobacter pylori infection. Gastroenterología y Hepatología. 2017;40 :651-657. DOI: 10.1016/j.gastrohep.2017.07.007 - 59.
McNulty CA, Wyatt JI. ACP. Best practice no 154. February 1999. Helicobacter pylori . Journal of Clinical Pathology. 1999;52 :338-344. DOI: 10.1136/jcp.52.5.338 - 60.
Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, et al. Helicobacter pylori infection. Nature Reviews. Disease Primers. 2023;9 :19. DOI: 10.1038/s41572-023-00431-8 - 61.
Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: The polymerase chain reaction. Cold Spring Harbor Symposia on Quantitative Biology. 1986; 51 :263-273. DOI: 10.1101/sqb.1986.051.01.032 - 62.
Mullis KB. The unusual origin of the polymerase chain reaction. Scientific American. 1990; 262 :56-65. DOI: 10.1038/scientificamerican0490-56 - 63.
Denninghoff V. Molecular pathology in the new age of personalized medicine. In: Pathology—From Classics to Innovations. London, UK, London, UK: IntechOpen; 2021. DOI: 10.5772/intechopen.94927 - 64.
Crossley BM, Bai J, Glaser A, Maes R, Porter E, Killian ML, et al. Guidelines for sanger sequencing and molecular assay monitoring. Journal of Veterinary Diagnostic Investigation. 2020; 32 :767-775. DOI: 10.1177/1040638720905833 - 65.
Lenze D, Müller HH, Hummel M. Considerations for the use of formalin-fixed and paraffin-embedded tissue specimens for clonality analysis. Journal of Hematopathology. 2012; 5 :27-34. DOI: 10.1007/s12308-012-0138-8 - 66.
Mathieson W, Thomas GA. Why formalin-fixed, paraffin-embedded biospecimens must be used in genomic medicine: An evidence-based review and conclusion. The Journal of Histochemistry and Cytochemistry. 2020; 68 :543-552. DOI: 10.1369/0022155420945050 - 67.
Greer CE, Lund JK, Manos MM. PCR amplification from paraffin-embedded tissues: Recommendations on fixatives for long-term storage and prospective studies. PCR Methods and Applications. 1991; 1 :46-50. DOI: 10.1101/gr.1.1.46 - 68.
Hammar M, Tyszkiewicz T, Wadström T, O'Toole PW. Rapid detection of Helicobacter pylori in gastric biopsy material by polymerase chain reaction. Journal of Clinical Microbiology. 1992;30 :54-58. DOI: 10.1128/jcm.30.1.54-58.1992 - 69.
Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985; 230 :1350-1354. DOI: 10.1126/science.2999980 - 70.
Oshowo A, Tunio M, Gillam D, Botha AJ, Holton J, Boulos P, et al. Oral colonization is unlikely to play an important role in Helicobacter pylori infection. The British Journal of Surgery. 1998;85 :850-852. DOI: 10.1046/j.1365-2168.1998.00724.x - 71.
Osaki T, Matsuki T, Asahara T, Zaman C, Hanawa T, Yonezawa H, et al. Comparative analysis of gastric bacterial microbiota in Mongolian gerbils after long-term infection with Helicobacter pylori . Microbial Pathogenesis. 2012;53 :12-18. DOI: 10.1016/j.micpath.2012.03.008 - 72.
Lee YC, Dore MP, Graham DY. Diagnosis and treatment of Helicobacter pylori infection. Annual Review of Medicine. 2022;73 :183-195. DOI: 10.1146/annurev-med-042220-020814 - 73.
Hu Y, Zhu Y, Lu NH. Novel and effective therapeutic regimens for Helicobacter pylori in an era of increasing antibiotic resistance. Frontiers in Cellular and Infection Microbiology. 2017;7 :168. DOI: 10.3389/fcimb.2017.00168 - 74.
Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016;65 :870-878. DOI: 10.1136/gutjnl-2015-311019 - 75.
Gisbert JP. The recurrence of Helicobacter pylori infection: Incidence and variables influencing it. A critical review. The American Journal of Gastroenterology. 2005;100 :2083-2099. DOI: 10.1111/j.1572-0241.2005.50043.x - 76.
Lee JS, Kwon KA, Jung HS, Kim JH, Hahm KB. Korea red ginseng on Helicobacter pylori -induced halitosis: Newer therapeutic strategy and a plausible mechanism. Digestion. 2009;80 :192-199. DOI: 10.1159/000229997 - 77.
Zhang L, Chen X, Ren B, Zhou X, Cheng L. Helicobacter pylori in the oral cavity: Current evidence and potential survival strategies. International Journal of Molecular Sciences. 2022;23 :13646. DOI: 10.3390/ijms232113646 - 78.
Katelaris P, Hunt R, Bazzoli F, Cohen H, Fock KM, Gemilyan M, et al. Helicobacter pylori world gastroenterology organization global guideline. Journal of Clinical Gastroenterology. 2023;57 :111-126. DOI: 0.1097/MCG.0000000000001719 - 79.
Aziz Y, Rademacher WMH, Hielema A, Wishaw SBP, van Diermen DE, de Lange J, et al. Oral adverse effects: Drug-induced tongue disorders. Oral Diseases. 2021; 27 :1528-1541. DOI: 10.1111/odi.13680 - 80.
Li Y, Choi H, Leung K, Jiang F, Graham DY, Leung WK. Global prevalence of Helicobacter pylori infection between 1980 and 2022: A systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology. 2023;8 :553-564. DOI: 10.1016/S2468-1253(23)00070-5 - 81.
Elbehiry A, Marzouk E, Aldubaib M, Abalkhail A, Anagreyyah S, Anajirih N, et al. Helicobacter pylori infection: Current status and future prospects on diagnostic, therapeutic and control challenges. Antibiotics (Basel). 2023;12 :191. DOI: 10.3390/antibiotics12020191 - 82.
Rolfo C, Denninghoff V. Globalization of precision medicine programs in lung cancer—A health system challenge. The Lancet Regional Health - Europe. 2023; 36 :100819. DOI: 10.1016/j.lanepe.2023.100819 - 83.
Usui Y, Taniyama Y, Endo M, Koyanagi YN, Kasugai Y, Oze I, et al. Helicobacter pylori , homologous-recombination genes, and gastric cancer. The New England Journal of Medicine. 2023;388 :1181-1190. DOI: 10.1056/NEJMoa2211807 - 84.
Paes Dutra JA, Gonçalves Carvalho S, Soares de Oliveira A, Borges Monteiro JR, Rodrigues Pereira de Oliveira Borlot J, Tavares Luiz M, et al. Microparticles and nanoparticles-based approaches to improve oral treatment of Helicobacter pylori infection. Critical Reviews in Microbiology. 2023;28 :1-22. DOI: 10.1080/1040841X.2023.2274835 - 85.
Noszka M, Strzałka A, Muraszko J, Kolenda R, Meng C, Ludwig C, et al. Profiling of the Helicobacter pylori redox switch HP1021 regulon using a multi-omics approach. Nature Communications. 2023;14 :6715. DOI: 10.1038/s41467-023-42364-6